chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Reduction of helional to its amine
Out of curiosity I recently used some aluminum amalgam in MeOH with aqueous ammonia solution to reduce helional. Before you lecture me on how low
yielding Hg/Al reduction are with ammonia solution, yes, I am aware. I ended up with under 200mg of what i think is my product.
The reduction went swimmingly, and since i started with only 5g of Ketone, the grey sludge build up didn’t hinder magnetic stirring. most the
aluminum was gone after 8 hours.
To work it up I added around a gram of KOH and stirred it in, and then I extracted the mixture with ether 3 times. I washed the combined extractions
with saturated salt solution a few times with venting and then dried the ether with anhydrous magnesium sulfate. I poured off the dried ether extract
and extracted it with very dilute H2SO4 solution 3 times with venting, and then i had to attend to other business so i set aside the aqueous layer
which should have my product. I also wanna note that when the sulfuric acid was added, a yolk-like sticky fluid appeared. This sucked and i have no
idea what it is but methanol and acetone seemed to work really well for getting it out of my funnel.
Anyways, after letting the aqueous layer sit out for a while, i noticed a layer of white-pink substance stuck to the bottom of the beaker holding it,
so i scraped it off to get flakey slightly pink crystals. I filtered the solution and let the crystals dry out.
The result is around 200mg of a white/pink, fluffy, very fragrant powder which i assume is the amine. It smells just like helional but with a sharp
“chemical” odor along with it.
I would just call this the end of smooth but low yielding reduction, but yet i cant help but wonder what the yolky liquid was. It was just like what i
got at my first attempt synthesizing helionamide via hydroxylamine/helional/H2O2 in DMSO.
Ive been told by some folks that this is the nitrile, which I believe, but if thats the case then how the hell did it form? I’m not aware of any
nitrile formation in aluminum amalgam reductions.
Ive attached an image of a rough reaction scheme for anyone who wants to see.
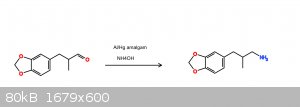
|
|
|
SaccharinSlayer157
Harmless

Posts: 26
Registered: 1-10-2020
Location: Near Cou?
Member Is Offline
Mood: Resisting the urge to build a ketene lamp
|
|
I would be very interested to see you succeed with this synthesis as I recently got access to both a small amount of the aldehyde and some elemental
mercury. Hopefully the other forum guys can help work out the kinks!
[Edited on 7-10-2020 by SaccharinSlayer157]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You probably produced some helionol (the alcohol) which formed a surfactant-like alkyl sulfate with the H2SO4. That would be my guess, at least.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by SaccharinSlayer157  | I would be very interested to see you succeed with this synthesis as I recently got access to both a small amount of the aldehyde and some elemental
mercury. Hopefully the other forum guys can help work out the kinks!
[Edited on 7-10-2020 by SaccharinSlayer157] |
Why though, it is still the one carbon too long inactive compound?
|
|
|
SaccharinSlayer157
Harmless

Posts: 26
Registered: 1-10-2020
Location: Near Cou?
Member Is Offline
Mood: Resisting the urge to build a ketene lamp
|
|
I think compounds that are super close to others with WILDLY different effects are super interesting. For example, it blows my mind how
L-Methamphetamine and phenyltertbutylamine are both close isomers of street meth while their effects are completely tame in comparison. Naltrexaone
is another good example from a different class.
[Edited on 7-10-2020 by SaccharinSlayer157]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 350
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Do something useful with your Helional man! Change it into MDA doing a Beckman and Hoffman rearrangement! Take a look at a recipe attached:
Attachment: Helional to MDA.doc (29kB)
This file has been downloaded 1414 times
[Edited on 7-10-2020 by Chemi Pharma]
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Quote: Originally posted by Chemi Pharma  | Do something useful with your Helional man! Change it into MDA doing a Beckman and Hoffman rearrangement! Take a look at a recipe attached:
[Edited on 7-10-2020 by Chemi Pharma] |
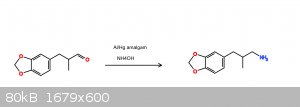
Have you actually ever tried any of this? I have. Using Sodium hypochlorite(bleach) as your halogen source doesn’t work very well for the Hoffman on
helionamide specifically, TCCA works better.
Even if it did, I’ve never heard of the nickel acetate method being performed successfully.
I have however had one success with a one-pot synth of the amide using hydroxylamine and helional in DMSO with H2O2. The reaction is very temperature
and pH specific, so its difficult to get right.
If you do it right, you get flakey white crystals of the Amide after the work up.
If you do the basic H2O2 oxidation wrong, you get this awful yolky liquid that is incredibly sticky. I’m told its the nitrile intermediate which
didn’t form into the amide because of too low temperature and wrong pH.
Idk, if anyone knows anything about this(not Karlos3), please do tell.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | | You probably produced some helionol (the alcohol) which formed a surfactant-like alkyl sulfate with the H2SO4. That would be my guess, at least.
|
Last time i checked, alkyl sulphate formation via an alcohol+H2SO4 routes required high temperatures and nearly anhydrous reagents. Are alkyl
sulphates typically sticky and viscous? And if such alkyl sulphate was a surfactant, i pity its strength as one due to no emulsion forming whatsoever.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 350
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Do you know about a study by Samuel Chill and Robert Mebane telling about one pot conversion of aldehydes into amides using hydroxilamine
hydrochloride and DMSO at 100ºC to make the nitrile and further hydrolysis in situ into the amide with NaOH and H2O2? I'm attaching that. Maybe could
be useful to you or another members.
Attachment: aldehydes to nitriles and amides - NH2OH + DMSO + H2O2.pdf (167kB)
This file has been downloaded 732 times
[Edited on 7-10-2020 by Chemi Pharma]
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Yes, I have seen that. There is an entire thread on the vespiary if u wanna take a look, but what I’m looking for is personal experience, really.
|
|
|
SaccharinSlayer157
Harmless

Posts: 26
Registered: 1-10-2020
Location: Near Cou?
Member Is Offline
Mood: Resisting the urge to build a ketene lamp
|
|
https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/in...
Here's a thread claiming success on some fronts but overall the metal salt process still looks undesirable.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Quick update since I didn’t want to start a new thread:
Yes, the nickel acetate method does in fact work. I tried it using 10g of oxime and 30ml of xylene, reflux for 7 hours at 134C with stirring, distill
off half of the xylene, let cool, filter off the amide and recrystallize 4 times in hot 10% isopropanol.
It was an off white by then end. the Hoffman works, too, but i only got 10% yeild using 6% bleach.
Thanks twodogs! 
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Quote: Originally posted by Chemi Pharma  | Do something useful with your Helional man! Change it into MDA doing a Beckman and Hoffman rearrangement!
[Edited on 7-10-2020 by Chemi Pharma] |
That's what I call encouragement  , ,
|
|
|