Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
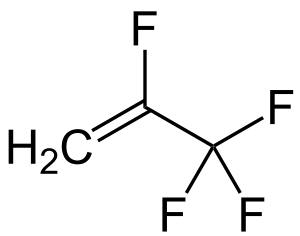
TFA via oxidation of R-1234yf?
So out of curiosity I wanted to see if there was any easy way to obtain TFA without having to deal with HF and I found an old post about oxidizing
R-143 which is 1,1,1-trifluoroethane. Through a little bit of reading I found out that R-143 is being phased out for another refrigerant under the
name R-1234yf which just so happens to be 2,3,3,3-tetrafluoropropene. This is itself just trifluoroacetyl fluoride with a methylene group instead of
an oxygen. Could this not be easily oxidized to trifluoroacetyl fluoride? Now the HF produced by the hydrolysis to TFA is a bit discerning but using
basic KMnO4 should mop up the HF no?
I don’t have plans on performing this anytime soon as I am much too busy however I have been looking at amine oxidations and TFPAA is listed just
about everywhere at the oxidizer of choice and even though I was planning on just using performic acid, having another available, easy to come, and
useful reagent would be appreciated
R-1234yf (below)
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
If you are looking for cheap reagents i think air is pretty good. Im planning trying to go for TFA and/or TFA-Chloride with R-134
(1,2,2,2-tetrafluoroethane). I think it has much higher chance of success than R-143 because the hydrodecarboxylation too trifluoromethane is not
possible. Hopefully copper or something similar will catalyse the reaction with air. But if you really want to use R-123yf maybe try ozone, it should
be next best thing to air in price and ozonolysis of alkenes is well documented.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
I like the ozonolysis idea. It looks like that can be formed at -78C which would be below the boiling point of R-1234yf. This could be performed in
methanol with a bit of NaOH to neutralize any HF produced or at the very least, hydrolyze any trifluoroacetyl fluoride into TFA. Definitely one to log
away for later. It’s kind of scary though when dealing with the possibility of HF as a product
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
HF + ozone + fluorocarbon + cryogenic temperatures
Sounds like a party!
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You should use WO3/H2O2 for this cleavage, not ozone or permanganate. It's simply much safer, which is especially critical when dealing with
fluorostuff. See e.g.:
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
clearly_not_atara that definitely is an interesting oxidation procedure with tungstic acid and hydrogen peroxide. The only concern I have is that
R-1234yf is a gas above -30C and hydrogen peroxide is a solid below -2C. I’m not sure how this oxidation reaction would work with a solid aqueous
phase.
[Edited on 1-12-2020 by Opylation]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
you could add an inert solvent to form a eutectic
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
You're totally right, I wasn't thinking about the H2O-H2O2 mixture freezing point which at 40% is about -40°C. This is very interesting indeed. I'm
going to have to put this on my to-do list
|
|
|