| Pages:
1
2 |
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Ethanol Distillation
Hi all,
So I am wanting to distill some ethanol in the future. I was thinking of using a 5,000mL round bottom flask
However, I'm unsure as to how to heat this big of a flask and what should I do about bumping?
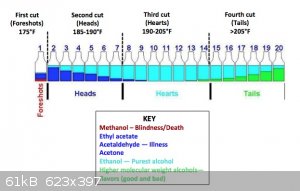
I found this website, which seemed to be helpful, it said how much of the first cut to throw out.
https://www.acsh.org/news/2017/06/06/throw-away-first-cut-po...
Lastly, how much 40% alcohol can I get if I fill the flask... say 2/3rds full and start with say 17% alcohol?
Any tips or advice? - Do I really want to increase the temperature past the boiling point of alcohol? Is that even possible when alcohol is in the
water? ( see depiction)
Suggestions welcome
[Edited on 8/17/2020 by Yttrium2]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
It's pretty easy to do. I strongly recommend using a column. You can get by with something simple like a 30 cm Vigreux, but you'll have to do a few
distillations to obtain azeotropic ethanol.
For heating, you can use a water bath.
You probably want to first do a stripping run with a pot still to remove the ethanol from the fermentation mixture and then purify the ethanol further
by distillation and treatment with chemicals like potassium carbonate and sodium metabisulfite, which are sold at brewing stores (oddly, not for that
intended purpose).
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Ethanol boils at a low temp and doesn't bump,. it cleanly boils like water if you use a stirbar.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
For 4L flask, I filled it to 3.5 liters when I was purifying 95% car alcohol. I started the distillation slower to prevent it from foaming to the
still head, but I never had issues with bumping. Use CaCl2 bath to immerse the flask into for even and stable heating. Water bath creates only 20C
gradient at best, IF you get azeotropic distillation at 78-ish C. Without reflux, you will be taking it between 84 to 90C and the distillation will be
exceedingly slow and yet result 40-60% at max. Any lab hotplate is unlikely to be able to heat that mass sufficiently to keep the distillation at good
speed with any flask above 1L, so get an actual 2kW hotplate or use gas burner to heat the CaCl2 bath. I prefer induction plate. You will want to get
lots of heat, which is explained below. Use active carbon granules for boiling stones if necessary.
For 17% wash, you'll get maximum of ~150mL of ethanol per every liter.
I prefer stripping it first with fast full blow distillation to bring it to 40-60% and then do the fractioning. This is especially needy if you have
small boiler.
For getting azeotrope, you will need active reflux and a long column, likely 500mm and above to get reasonable separation speed. Get a vigreux or a
column you can pack with raschig rings or SS wool, a reflux condenser, claisen head and and a valve adapter to adjust the reflux ratio. You will need
1:10 reflux to get to azeotrope, meaning, for every mL you return 10mL back to the column. You can also replace the claisen head with dean-stark or
clevenger, so the enriched product will concentrate on the receiver and you'll be able to get good separation. You will allow the product to pool in
the head and only collect it so that the reflux ratio remains, never drain it. Don't adjust the ratio with heating - use as much heating power as the
column can handle without choking, the more reflux you get the better separation there will be. Easiest way to determine concentration is to measure
the head temperature. Azeotrope sits at 78-79C depending on setup and barometric pressure, but when the equilibrium is disturbed or the feedstock is
getting depleted, it will quickly shoot to +84C. Collect in 1-2dL increments in glasses as your picture states. Generally the range of 78-84C is
collected for potable run, in which you'll get following:
- Foreshots, which contain lower boiling point liquids from fermentation like acetone, methanol, ethyl acetate, etc. about 5% of total distillation
volume
- Heads, which is a blend of former and also a major amount of ethanol - this is already suitable for low end chemical applications and solvent use,
about 20%
- Hearts, which is generally regarded as the potable portion, about 40-50% depending how conservative you are
- Tails, which contains an increasing amount of heavier alcohols, aka fusel oils and give the body to the aged alcohols like whiskey. You'll
eventually see a film of oil forming on surface and the smell gets progressively stronger and eventually turns into wet dog - cardboard like. About
30-50%, depending on how much body is preferred
For chemical uses, unless a very pure ethanol is necessary, generally everything except the first few of the heads and last few of the tails can be
used. For very high grade ethanol, only collect hearts and distill it with carbonate and polish it with activated carbon, and finally dry it with
proven methods if anhydrous is necessary.
For chemical purposes a turbo yeast is good because it can get 17, some fuel industry strains even 22+% ABV wash, but they are absolutely not usable
for potable alcohol production, unless scrupulous purification methods are applied. The little impurities make it taste like rocket fuel with a hint
of goat dung.
For stripped liquid of 40-60%, you can use (sodium) carbonate or even hydroxide at 5g/L to kill residual acetates, acetic acid and other similar
impurities. Never mix this with the fermentation wash, because basifying the ferment will make ammonia leach out. For azeotropic distillate, activated
carbon can be used to polish it even further. This though is best done when diluted to 50-60% if potability is a matter. Carbon can be regenerated by
rinsing, boiling until no detectable odor evolves and then heating in oven at max power (usually 250-300C).
I made moonshine for personal use times long gone and I went through all the details to get the setup actually working. The fractional distillation
procedure is directly usable for all other chemistry purposes and the setup is routine in chemical industry. Vigreux column as an air cooled device is
mostly suitable only for experimental sample distillations where time and resources does not have to correlate with outcome. For distilling any usable
quantity of ethanol, you'll easily use 12h, 24h or even longer runs to get even a liter or two of azeotrope. A bokakob still with 75mm column diameter
by 1000mm packing height will get around 600-900mL of azeotropic ethanol per hour with 5kW propane heater. With immersion or induction heater,
insulating the boiler and column the efficiency could be significantly improved, though.
|
|
|
MadHatter
International Hazard
    
Posts: 1339
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Method
Fyndium, your method is certainly sound.
In the case of my old still I put a thermometer
in the top of the reflux column. Anything that
came over before 78C was tossed. I shut
the still down when the temperature hit 80C.
I'm sure I didn't have the efficiency that you've
described.
As for making it anhydrous, I ran it through
dehydrated rock salt. NaCl doesn't dissolve
very well in C2H5OH. That got most if not
all of the water out for me.
Picture of my now gone Destilabs still.
Note: Thermometer not shown.
http://www.sciencemadness.org/talk/viewthread.php?tid=2008#p...
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Fyndium  | For 4L flask, I filled it to 3.5 liters when I was purifying 95% car alcohol. I started the distillation slower to prevent it from foaming to the
still head, but I never had issues with bumping. Use CaCl2 bath to immerse the flask into for even and stable heating. Water bath creates only 20C
gradient at best, IF you get azeotropic distillation at 78-ish C. Without reflux, you will be taking it between 84 to 90C and the distillation will be
exceedingly slow and yet result 40-60% at max. Any lab hotplate is unlikely to be able to heat that mass sufficiently to keep the distillation at good
speed with any flask above 1L, so get an actual 2kW hotplate or use gas burner to heat the CaCl2 bath. I prefer induction plate. You will want to get
lots of heat, which is explained below. Use active carbon granules for boiling stones if necessary.
|
I am completely baffled as I read this... I've distilled ethanol with a water bath, no CaCl2, with a regular lab hotplate lots of times, usually in a
3L flask, with a column. The recovery is not 100%, but it definitely works, and you can strip the volatile components left behind and redistill to
reduce waste. It does take kind of a long time, but it's fast enough to produce a few liters per day if you actually need that much for some reason.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
I use Alcotec Turbo yeast, which consistently brings me a yield of about 200ml's of >90% EtOH per liter of wash after fermenting about two weeks.
Costs about $20 for about 2/3'd's gallon "pure grain alcohol"
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
I'd recommend a metal vessel if possible as a glass flask over a steam bath is going to take forever and it's already gunna take forever.a hotplate
with a modified cooking pot would be faster.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
If you would choose a glass vessel, I would advice to use a sand bath. A sand bath is as simple as sand in a pan, you can then quite safely use a
flame or a electric plate to heat the distillation. If anything breaks the alcohol will be contained by the sand instead of spilling everywhere or
boiling vigorously in case of an oil bath.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
YMMV.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
A water bath is automatically the right temperature....
If you don't want to use a water bath, you might as well use a heating mantle. Ethanol doesn't usually bump, but it can, and bumping is detrimental to
the purity, so I advise adding a PTFE stirbar before applying heat.
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Fyndium  | | For 4L flask, I filled it to 3.5 liters when I was purifying 95% car alcohol. I started the distillation slower to prevent it from foaming to the
still head, but I never had issues with bumping. Use CaCl2 bath to immerse the flask into for even and stable heating. Water bath creates only 20C
gradient at best, IF you get azeotropic distillation at 78-ish C. |
Can you explain the CaCl2 bath? I've never heard of this and I'm guessing it's a high concentration of CaCl2 in solution. CaCl2 has a very high
solubility, especially in hot water and I'm guessing the reason is because it increases the density or heat capacity of the solution/water?
What % of CaCl2 do you use and is there anything specific you do? Are there other salts that can be used in place of this and would any other salts
maybe be better or offer a higher heat capacity?
The only problem I can possibly see is with the displacement of the flask and having to weigh it down or make sure the clamp is very tight as the more
ethanol is distilled, the more the flask will want to rise up, so you might think it is secure, but find our later on that the flask is floating to
the top or worse if you don't have it secured correctly.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
CaCl2 bath is just plain and simple calcium chloride dissolved in water. Concentration? I have honestly no idea, and I don't neither care that much,
as long as it does the job. It costs 40 cents per kg. I dissolved it by hand to get the required volume to fill my kettle which I heat with induction
plate. The melting point of CaCl2 solution rises steeply when the concentration goes above 50-60%, so this should be used mostly when a hot bath is
needed. CaCl2 can go as high as 170-180C while remaining liquid, but the boiling temp hovers around 140-150C when the solution is still liquid in a
warm room. Benefit to water? It evaporates very slowly, does not boil, does not burn like oil and it is pretty much odorless.
Quote: Originally posted by JJay  | | I've distilled ethanol with a water bath, no CaCl2, with a regular lab hotplate lots of times, usually in a 3L flask, with a column.
|
Yes, it works, but it's slow. You don't even have to boil the substance to make it evaporate so you can collect it. Short path distillation apparatus
can be used for this very purpose.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Why would you use a the sticky CaCl2 instead of sand? Every drop of CaCl2 spilled will form a sticky mess that doesn't evaporate.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Fyndium  | CaCl2 bath is just plain and simple calcium chloride dissolved in water. Concentration? I have honestly no idea, and I don't neither care that much,
as long as it does the job. It costs 40 cents per kg. I dissolved it by hand to get the required volume to fill my kettle which I heat with induction
plate. The melting point of CaCl2 solution rises steeply when the concentration goes above 50-60%, so this should be used mostly when a hot bath is
needed. CaCl2 can go as high as 170-180C while remaining liquid, but the boiling temp hovers around 140-150C when the solution is still liquid in a
warm room. Benefit to water? It evaporates very slowly, does not boil, does not burn like oil and it is pretty much odorless.
Quote: Originally posted by JJay  | | I've distilled ethanol with a water bath, no CaCl2, with a regular lab hotplate lots of times, usually in a 3L flask, with a column.
|
Yes, it works, but it's slow. You don't even have to boil the substance to make it evaporate so you can collect it. Short path distillation apparatus
can be used for this very purpose. |
No way. You simply cannot expect to obtain azeotropic ethanol from an aqueous solution without boiling it. Regardless, ethanol boils on a water bath.
You're going to have to go slow to obtain azeotropic ethanol from a water mixture no matter what heating method you use unless you use a large column.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I wasn't speaking specifically of alcohol on enhanced evaporative distillation. It is generally more usable for higher boiling point high value
reagent extractions where the volume and speed is not essential but limited decomposition and/or other requirements must be fulfilled. Ethanol is
difficult to make azeo without reflux distillation, or at least the efficiency is low. With reflux, I prefer the fast way: make it boil like hell to
generate as much vapor as the column can handle, and adjust the reflux ratio to reach azeotrope equilibrium.
Ethanol from low abv wash boils at much higher temp than pure ethanol. Please refer to attachment. Boiling 90C liquid(17abv) with 100C bath is just
slow. Even the literature suggests having at least 20-30C gradient on bath for distillations.
Column height has only air convection to control the reflux equilibrium, which creates bad efficiency. Ideal column (or at least in industry) is
vacuum insulated so the only gradient comes through refluxing. Insulated (vacuum) reflux column can be used to produce very high purity distillates
with good control on temp, because the reflux acts as a buffer.
Sand has very low thermal conductivity. It is even slower than many other methods. It also tends to burn out electric hotplates due to insulation,
dunno about induction. It may also scratch glass, but salt is no better on thermal properties. CaCl2 bath is close to water in conductivity - and did
I already mention, it does not boil, so it does not splatter. It just basically sits there, and during 8-hour distillation run, the evaporation had
reduced surface by only 2cm.
The salty residue is also very easy to just rinse off with water, being highly soluble and it is easy to wipe it dry to handle the flasks what is
needed - and you can also dip them in proper temp water to quickly remove all salt. Oil bath is a horror story compared to this.
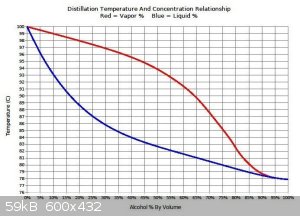
[Edited on 17-8-2020 by Fyndium]
[Edited on 17-8-2020 by Fyndium]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
As I previously mentioned, it's a lot faster to use a pot still to do a stripping run before you purify the distillate in laboratory glassware on a
water bath. You're not going to have a choice about doing a stripping run really unless you have enormous glassware or make very small amounts of
ethanol.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
For those of you who have never seen a pot still, here's a commercial one: https://www.amazon.com/dp/B07TGKDY4F/ref=sspa_dk_detail_3
Nile Red made one: https://www.youtube.com/watch?v=m2DfCr2Qsx0
You can put one together without doing any soldering with a pressure cooker, some fittings, and copper tubing, along with a regular laboratory
condenser.
Home distillation is illegal in the U.S., but it's been decriminalized in many places. People actually do get arrested for bootlegging on occasion, so
check your local laws before you distill any ethanol.
|
|
|
MadHatter
International Hazard
    
Posts: 1339
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Still
Attachment: The_Spiral_still.pdf (926kB)
This file has been downloaded 540 times
The above pdf may be of interest to some.
Arkoma, years ago I used a yeast called
Alltech SuperStart. Can't seem to locate it
anymore. Maybe it's the Alcotec that you
use now. Lalvins K1 and EC-1118 also did
a good job. Like your Alcotec, the SuperStart
could produce ABV at 20 - 21%. It was
fun watching it ferment because the mash
looked like it was boiling.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Quote: Originally posted by JJay  | | As I previously mentioned, it's a lot faster to use a pot still to do a stripping run before you purify the distillate in laboratory glassware on a
water bath. You're not going to have a choice about doing a stripping run really unless you have enormous glassware or make very small amounts of
ethanol. |
Absolutely. I always did stripping first and then combined the batches to commerce a single reflux distillation operation. This results in a more pure
product with better efficiency.
I personally prefer the boka design. It is extremely simple and efficient and it could be done out of copper by soldering with rudimentary tools, or
welding from SS.
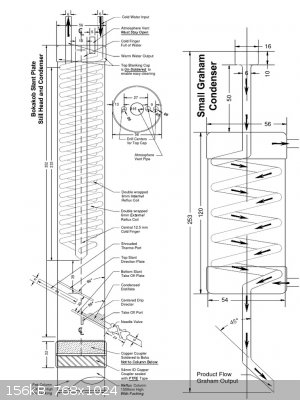
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tsjerk  | | Why would you use a the sticky CaCl2 instead of sand? Every drop of CaCl2 spilled will form a sticky mess that doesn't evaporate.
|
You can wipe up sand with a damp cloth,wherease cleaning up CaCl2 needs... a damp cloth.
However the effective thermal conductivity of the liquid is much higher (because you get convection currents).
That means you get better temperature control.
For what it's worth a small bit of wood- a matchstick or something weighted with a bit of copper wire so it stays at the bottom of the flask is a
very effective anti bumping agent.
Useless in corrosive environments, but pretty much inert to alcohol + water.
Use a few- they are cheap and you really don't want a litre or more of flammable stuff bumping.
With good anti bumping you don't need a stir bar.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I've found activated carbon a decent anti bump agent. Inert to mostly everything, perhaps excluding HNO3 and some strong oxidizers, heavy enough to
stay at the bottom, as porous as you can get, easy to separate and also otc, cheap and ready to use straight from the bag.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I almost never, especially not for the last years, have distilled alcohol produced from fermentation.
If I produced pure ethanol, I do that starting from the cheap denat. alcohol in my country(which consists of 1% MEK, 96% ethanol, and some tiny amount
of bitrex).
This is much cheaper than all the sugar needed to make undenatured alcohol via yeast.
I take that stuff, reflux it with a little bit of NaOH(to produce a higher boiling product from the reaction of 2 or more MEK molecules via aldol).
And then, I simply distill that carefully over, which is very easy and quite quick too.
The distillate is distilled once again, and I'm left with very pure ethanol distillate afterwards.
No fusel oils will contaminate the product either this way.
And, as I've admitted somewhere already, I have even tried to drink some of this purified ethanol, diluted with coke, and it just tasted like almost
nothing, like pure grain or potato alcohol.
Actually, a real vodka would have tasted much stronger and apparent, in my opinion, than the purified EtOH from the denat. ethanol.
This workup method is clean and golden!
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
I've used stainless scubbers to pack columns before (2 & 3") and this place has the best ones I've ever found, they are huge and you can probably
pack a few glass columns with one scubber. They are 2-3x larger than ones at the store and 1/4-1/3 the price. The site also has some other
interesting things at good prices too. I've found a number of uses for the scrubbers as well and am glad I got 2 packs of them!
https://www.sciplus.com/6pack-100gram-heavy-duty-pot-scrubbe...
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Quote: Originally posted by karlos³  | I almost never, especially not for the last years, have distilled alcohol produced from fermentation.
If I produced pure ethanol, I do that starting from the cheap denat. alcohol in my country(which consists of 1% MEK, 96% ethanol, and some tiny amount
of bitrex).
This is much cheaper than all the sugar needed to make undenatured alcohol via yeast.
I take that stuff, reflux it with a little bit of NaOH(to produce a higher boiling product from the reaction of 2 or more MEK molecules via aldol).
And then, I simply distill that carefully over, which is very easy and quite quick too.
The distillate is distilled once again, and I'm left with very pure ethanol distillate afterwards.
No fusel oils will contaminate the product either this way.
And, as I've admitted somewhere already, I have even tried to drink some of this purified ethanol, diluted with coke, and it just tasted like almost
nothing, like pure grain or potato alcohol.
Actually, a real vodka would have tasted much stronger and apparent, in my opinion, than the purified EtOH from the denat. ethanol.
This workup method is clean and golden! |
Yes, this is very true and I do the same. Denatured ethanol costs few euros per liter and it is easy to purify into a very good chemistry grade
ethanol. Most ethanol is produced in the same facilities by azeotropic distillation, and they supply it to fuel and beverage industry by the train
cart. They then further process it, and as someone said, the grade mostly depends on how much activated carbon is applied to polish it.
I haven't tasted it, but I'm not surprised if it was quite decent vodka. Alcoholics do drink these as substitute alcohols because of low price. It
tastes like hell, but it gets you drunk as hell with little money, so... This is the same exact reason methanol is strictly forbidden for consumer
products because someone will always attempt to drink it.
The boka still I made specifically for potable alcohol.
|
|
|
| Pages:
1
2 |