| Pages:
1
2 |
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
successful method for purifying anthranilic acid(quick report)
Hey, this is a quick report on a successful attempt of mine to purify anthranilic acid made from phthalimide via the Hoffman rearrangement.
There's nothing really special about it, but I figured it can't hurt posting it in here, just in case someone would find it useful 
---
Stuff you'll be needing:
1. Crude anthranilic acid
2. Soxhelt apparatus
3. n-Heptane(other alkanes might also work, although it seems reasonable to assume that those with higher b.p would work better[or at the very least
faster]).
The process:
Fill the Soxhelt apparatus with the crude anthranilic acid, add some n-heptane and start refluxing. That's about it.
If larger crystals are desired, you can add a bit of methanol(after the extraction is over) and slowly boil it off. But keep in mind that some of the
acid will react to form methyl anthranilate(evident by a grapy odor). Adding ether might be a better choice.
---
pictures & descriptions:
beginning: a brown powder(somewhat sticky)
a bit after the beginning:

end:

extracted product(after recryst with methanol):

slower evaporation of the methanol:

---
full disclosure:
a relatively pure product can be obtained straight from the formation of the acid via the Hoffman rearrangement, if done meticulously.
Unfortunately, stuff like traces of phthalic anhydride/acid in your phthalimide, or uncertainty in the concentration of your hypohalite solution,
might make it a difficult goal to achieve.
[Edited on 7-7-2020 by B.D.E]
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
I'm surprised that works so well. Useful to know.
|
|
|
Tsjerk
International Hazard
    
Posts: 3035
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Why would higher boiling alkanes work faster? The actual extraction is cold right? And how did you evaporate the heptane? I found it quit hard to get
rid off in the past.
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by Tsjerk  | | Why would higher boiling alkanes work faster? The actual extraction is cold right? And how did you evaporate the heptane? I found it quit hard to get
rid off in the past. |
ummm, I'm pretty sure that some of the product had ppt after the solution was cooled
down. I may check on it again later this day just to be sure.
The extraction was carried via a soxhlet apparatus at ~1atm, with rapid refluxing straight into the body of the extractor. Hotplate was set to 380C.
So definitely a hot extraction.
As for getting rid of the heptane, I actually didn't had to. I simply added the methanol directly into the heptane until all of the product had
dissolved, then boiled off the methanol, let the solution cool and filtered the product with a sinter funnel. The resulting filtrate was pretty much
all heptane, with only trace amounts of the product itself.
Also, at least from my experience, getting rid of excess heptane was never an issue. I just heat it to it's boiling point(or slightly below it) and
let it evaporate. It also evaporate relatively fast at rt with a fan on it.
[Edited on 8-7-2020 by B.D.E]
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Wow... very useful workup, I will try this for sure with my somewhat crude anthranillic acid. Hydrocarbons sometimes do wonders in terms of
purification.
Thanks for sharing B.D.E
One question: should cooling the heptane/AA solution in freezer crash most of the Anthranillic acid from the solution without the need to evaporate
excess of solvent?
[Edited on 8-7-2020 by mackolol]
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Glad to hear you find it useful 
As for your question, I just want to clarify that anthranilic acid is only very slightly soluble in heptane(roughly 1-2 grams per
liter at ~80C). (this is also why a soxhlet extractor is an absolute must-have for this workup).
So as long as you don't mind having a powdered end-product, you can just filter the heptane out at rt, right after the extraction is complete, without
a significant lost of yield.
[Edited on 8-7-2020 by B.D.E]
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Quote: Originally posted by B.D.E  |
So as long as you don't mind having a powdered end-product, you can just filter the heptane out at rt, right after the extraction is complete, without
a significant lost of yield.
[Edited on 8-7-2020 by B.D.E] |
All right, that's what I have thought.
In case of anthranillic acid, I don't see any other point in having bigger crystals, than just aesthetic matter.
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by mackolol  | | In case of anthranillic acid, I don't see any other point in having bigger crystals, than just aesthetic matter. |
yes, that's about it.
I just wanted a trophy 
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Nice, finally a use for my soxhlet apparatus! I remember an attempt at making anthranilic acid which gave me a beige powder, but even
recrystallization from ethanol didn't remove the beige color. This looks like it will work well!
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
So is this just a recrystallization of the aa straight out of the reaction of hypohalite and pthalimide? No copper salt purification? If so it seems
great bcoz the copper salt thing seems like a hassle.this seems like a great way to clean up a hard to clean product.apart from the glassware
requirement
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
And this is another reason to buy soxhlet exctractor, which I wanted to do for a long time but I'm always forgetting 
|
|
|
Benignium
Hazard to Others
  
Posts: 115
Registered: 12-6-2020
Member Is Offline
Mood: Quasi-catatonic
|
|
Great find and awesome writeup! Thank you!
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
I have about 65g of phthalimide and am planning on converting it to anthranilic acid as soon as I get the chance. Benignium suggested your soxhlet
extractor purification technique, seems like a great idea. Did you put the crude anthranilic acid right into the soxhlet extractor? Or did you purify
it with boiling water first, then follow up with the soxhlet extraction?
P.S. From the looks of the product, I assume it was the crude anthranilic acid, but I thought I would confirm before doing it myself.
[Edited on 18-1-2021 by SuperOxide]
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
Quote: Originally posted by B.D.E  | [...]
If larger crystals are desired, you can add a bit of methanol(after the extraction is over) and slowly boil it off. But keep in mind that some of the
acid will react to form methyl anthranilate(evident by a grapy odor). Adding ether might be a better choice.
[...] |
How about chloroform instead? Anthranilic acid is "very soluble" chloroform, whereas it's only "soluble" in ethanol and ethyl ether
(src Pubchem), but the specific solubility isn't detailed.
|
|
|
Texium
Administrator
       
Posts: 4667
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
This is fantastic, B.D.E. That is the cleanest looking anthranilic acid I have ever seen, even compared to commercial samples. I will definitely be
doing this with mine once I resume home chemistry.
Edit: one thing that would be good to include is your percent recovery. If the process is particularly lossy, it may not be worth using in most cases,
though if the losses are minimal then this would be really great.
[Edited on 2-8-2021 by Texium (zts16)]
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
Quote: Originally posted by Texium (zts16)  | This is fantastic, B.D.E. That is the cleanest looking anthranilic acid I have ever seen, even compared to commercial samples. I will definitely be
doing this with mine once I resume home chemistry.
Edit: one thing that would be good to include is your percent recovery. If the process is particularly lossy, it may not be worth using in most cases,
though if the losses are minimal then this would be really great.
[Edited on 2-8-2021 by Texium (zts16)] |
I agree. Maybe include how long you ran the soxhlet extractor for as well, and how much crude anthranilic acid you were processing for that run time?
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
I was doing another anthranilic acid synthesis today, getting enough so that I can get enough to put in my soxhlet extractor whenever I get the time
to try this out, and after I dropped the pH to 3.5 and the AA precipitated out, I couldn't believe the color, it was the best color I've gotten thus
far! Just a very light beige (I think I could get by calling it "off white" even).
 
I was thinking that maybe I wouldn't have to do the soxhlet extraction using heptane... Instead, I could just give it a quick recryst with some
boiling water...

This stuff is so damn touchy  How the hell does it get darker in the recryst?? How the hell does it get darker in the recryst??
Looks like I'll be trying this out after all. I might even skip the recryst on some of the runs and go right to the soxhlet trick detailed above.
P.S. Here's the other pictures I took, if anyones curious. And some other photos of the previous synthesis runs.
[Edited on 12-2-2021 by SuperOxide]
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
@B.D.E - Can you tell me how much crude anthranilic acid you processed and for how long you refluxed? I'm doing this right now, and I planned on
sharing my results. On the run I'm doing right now, I'm processing 10g of anthranilic acid with about 120mL of heptane (probably not n-heptane, since
it mostly distilled over at 91 °C, but it seems to work regardless).
I ran the soxhlet for probably about 7 hours straight, and while it's obvious that it worked by the amount of solids that were precipitating out in
the bottom flask (once the heptane got saturated), it wasn't as much as I had hoped. Did you just let it run for a full 24 hours or something?
Edit: And I think that if the goal is purity, then a recryst after the soxhlet/heptane treatment is definitely important. The
crystals were a light brown when they were in the soxhlet, and when they were precipitating out in the flask they were a weird looking yellow color:

Now they're pretty close to white (I think real chemists would call it "off white"):

Definitely an improvement :-)
[Edited on 8-3-2021 by SuperOxide]
|
|
|
Texium
Administrator
       
Posts: 4667
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
What was your percent recovery?
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
Unfortunately, not very much (wasn't able to weigh it yet)... And I had to
cut it short, so I need to process the heptane/methanol recryst solution before weighing, but once that's done I will weigh the crystals I get from it
then share the details.
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
Just an update - I gave this a go again yesterday, and while it technically works, it takes a loooooooong... long time.. Honestly it's not worth it
unless you can set it and forget it for like 24 or 48 hours, or you just need a small sample of a nice white crystalline anthranilic acid sample to
test something out on.
Procedure
I added 20g of anthranilic acid (which was made via the Hofmann rearrangement of phthalimide, then recrystallized in boiling H2O) to my
150mL soxhlet extractor, added the heptanes (extracted from Super Tech Starting Fluid, which distilled over at 91 °C - 93 °C, suggesting it's not n-heptane) and cranked up the temperature. I ran it for a
little over 12 hours. I timed how long it took to cycle in the soxhlet extractor, and it was aprox 7 min and 20 sec, so that means it cycled
approximately 100 times (give or take a couple considering as the anthranilic acid got extracted, there was more room for solvent in the soxhlet
apparatus and less room for it in the flask)
20g of recrystallized anthranilic acid

Acid placed in the soxhlet extractor (in a coffee filter to prevent clogging, which was a problem
before)

At the end of the 12 hours, there was a yellow solid that was forming in the boiling flask, which was sticking to the stir bar pretty strongly.
The light yellow solid that precipitated out and clung to the stir bar


Flask with yellow solid from a different angle (notice there seems to be quite a bit on the sides)

I took the remaining anthranilic acid out of the soxhlet apparatus, dried it and weighed it at 15.69g - meaning only 4.31g was extracted over 12
hours. Thats about 0.359g extracted every hour. Even if one were to assume that the extracted solid was pure enough to use (which it seems to need a
recryst, so not likely), this is still a pitiful yield. Obviously this means the soxhlet is absolutely required if you want to use this route.
From here I kinda messed up a bit. I decided to just filter off the yellow solid and do a recryst with water, which was a bad idea. The crystals were
also yellow, as opposed to white (or slightly off white) that I expected to see from my previous smaller scale shorter run (also mentioned in a
previous post above).
Recrystallizing the yellow anthranilic acid in boiling H2O seems to yield long needle like crystals,
still light yellow in color


Filtered off (still slightly wet, but they looked the same when dried)
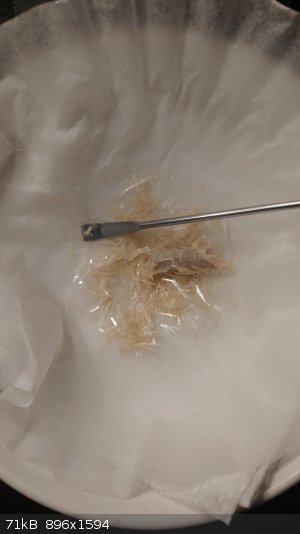
I did another recryst with MeOH which seems to have cleaned it up a bit more (though it's still in solution), but not as colorless as I was hoping.
Crystals of anthranilic acid starting to form in the MeOH as it cools

Same flask, but you can see some of the color seems to come off when the flask is tilted on its side and the
solvent is allowed to settle to the side (gives me hope it may clean up once filtered and dtried)
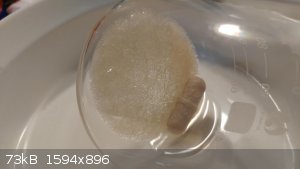

If anyones interested, I may post some photos of the filtered/dried crystals if they're any lighter in color.
This doesn't mean that a white solid can't be attained, since on a smaller scale, I added MeOH directly to the heptane/anthranilic acid mixture, then
heated it up with strong stirring and this yielded perfectly white crystals:
Crystals NOT from this run, just used as a comparison.

The fact that I was just messing up the recryst seems to be evident in that whenever I dissolve the anthranilic acid in MeOH, then pour it into a
different container after heating it up, the dribble that runs down the outside always turns out pure white!


So basically I messed up the recryst on this longer run by not just adding the MeOH directly to the heptane/anthranilic acid solution. Somehow that
yielded a much cleaner product - Perhaps the recryst from boiling H2O caused some oxidation due to the higher temperature? Not
sure about that.
Two things I may try next before running away with my tail between my legs:
- Running the soxhlet extraction using hexane instead of heptane - Will this be better? I have no idea. But the boiling point is
much lower than heptane (69 °C instead of ~93 °C). Perhaps lower BP = more cycles = more extraction? no idea.
- Try a multi-solvent recryst (of the brown crystals right from the Hofmann rearrangement of phthalimide) with heptane and diethyl
ether - Anthranilic acid is basically insoluble in heptanes, but very soluble in ether, so I'm just curious what will happen if I dissolve it
in some ether and crash it out with heptane.
Question for B.D.E: How long did you let the soxhlet extractor run? What was your yield? How long was your vacation you took in the
interim? (I'm assuming a month).
[Edited on 19-4-2021 by SuperOxide]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Water seems to be a no go for this substance. Going off everyone's before and after pictures water seems to make the stuff worse not better.
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
Quote: Originally posted by draculic acid69  | | Water seems to be a no go for this substance. Going off everyone's before and after pictures water seems to make the stuff worse not better.
|
Yep... I'm now convinced of that, 100%. I wish I could go back in time and slap myself before I made the stupid decision to use water (I tried to wash
the product with ice cold water, since it's much less soluble in ice cold water - still didn't work well). That's 12 hours, wasted, lol.
I may actually just try the whole synthesis again, and instead of using water when I recryst the crude product, I may use MeOH. The crude product in
my experience is almost white:

But when I use boiling water for the recryst, this is the same product:

So yeah, lets see if it keeps its color using MeOH as the solvent, keeping water out of it entirely.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Just a quick question; was tap or distilled water used? Maybe that's a factor maybe not.anyone have experience doing this with distilled vs tap water
my tap water has lots (LOTS) of crap in it. Maybe it's the water causing decomposition or oxidation
maybe it's gunk in the water? I mean cl2, fluoride substances, flocculant, carbonates of mg+ca, other minerals,copper steel or plastic traces from
piping etc are all
possibilities.
[Edited on 20-4-2021 by draculic acid69]
[Edited on 20-4-2021 by draculic acid69]
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
Quote: Originally posted by draculic acid69  | Just a quick question; was tap or distilled water used? Maybe that's a factor maybe not.anyone have experience doing this with distilled vs tap water
my tap water has lots (LOTS) of crap in it. Maybe it's the water causing decomposition or oxidation
maybe it's gunk in the water? I mean cl2, fluoride substances, flocculant, carbonates of mg+ca, other minerals,copper steel or plastic traces from
piping etc are all
possibilities. |
Great question - Yes, I tried it with both, no difference. I usually always use distilled water for recryst or any chemistry, and tap for cleaning
stuff. But I made sure to clean the beaker out with IPA and then use distilled water - still happened.
I just got some fresh bleach, I plan on making some anthranilic acid today or tomorrow, then trying out the recryst with a few different solvents
(MeOH, chloroform, ether, etc).
|
|
|
| Pages:
1
2 |