| Pages:
1
2 |
EliasExperiments
Hazard to Self
 
Posts: 57
Registered: 3-3-2020
Member Is Offline
|
|
Removing denaturing agents from alcohol
So I tried to make some pure ethanol by distilling some store bought ethanol. I show my process in this video:
https://youtu.be/p68HjVEqs4A
I think I was sucessfull, but I obviously have no good way to test if there is still isopropanol in there. Does anybody have an idea how I could
remove it, or how I could test for it?
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
The only way I can think of is fractional distillation, but it would be very inefficient.
Around here more and more ethanol is sold which doesn't contain methanol or isopropanol, it is sold as 99% bio-ethanol for fireplaces. Methanol is not
added anymore because it is toxic, in case someone would actually drink it, and IPA is not added because they don't have to.
This bio-ethanol only contains MEK and denatonium benzoate. Even the blue smelly (pyridine) stuff (85% ethanol) would be easier to clean than the IPA
denatured alcohol, as it only contains MEK, denatonium, pyridine and water. The pyridine can be easily contained in the distillation flask as the
sulfate salt.
The 99% ethanol can be easily made almost anhydrous by driving off the last bit of water as the azeotrope.
[Edited on 29-5-2020 by Tsjerk]
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Maybe benzene-ethanol azeotrope purification would work, but due to its carcinogenity it's rather not a way to go.
If you want to drink it try old slavic methods, pouring it through bread slice makes ambrosia from it   
|
|
|
Belowzero
Hazard to Others
  
Posts: 173
Registered: 6-5-2020
Location: Member Is Offline
Member Is Offline
|
|
Perhaps an unconventional approach but the freezing points of these alcohols are −114,4 °C ethanol and −89 °C for isopropyl.
Dry ice can reach -100 , it might be possible to freeze out the isopropyl , I have no idea how practical this is though.
I also came across this patent: https://patents.google.com/patent/US5445716A/en
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I'm pretty sure the IPA would dissolve in the ethanol giving them a shared freezing point. Also dry ice sublimes at - 79, not -100
[Edited on 29-5-2020 by Tsjerk]
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
if you freeze a 50/50% of ethanol and water you'll not get 100% liquid ethanol and 100%water ice. so the freezing thing is going yo be a huge waste of
energy.
in my country each brand of denaturated alcohol adds his own sruff, some add MEK, some add tert-butanol, others add whatever they have laying around
to make it hard to fractionally distill.
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Belowzero
Hazard to Others
  
Posts: 173
Registered: 6-5-2020
Location: Member Is Offline
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | I'm pretty sure the IPA would dissolve in the ethanol giving them a shared freezing point. Also dry ice sublimes at - 79, not -100
[Edited on 29-5-2020 by Tsjerk] |
Ah yes I suspected something like that would happen, the reason I mentioned it is that the freezing points are further apart than the boiling points.
As for the cooling bath , some sources list that an acteone/dry ice or IPA/dry ice bath can reach towards -100
[Edited on 29-5-2020 by Belowzero]
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
doubt as most organic synthesis that require cold temperatures will tell you to run the reaction at -78°C, simply because that's the temperature of a
dry ice/acetone bath
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
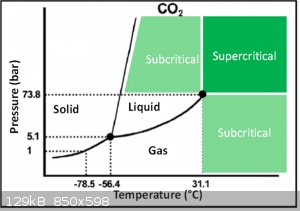
What are those sources? Basic physics say CO2 sublimes at -79 at ambient pressure. To get it to sublime lower you would have to lower the pressure.
|
|
|
Belowzero
Hazard to Others
  
Posts: 173
Registered: 6-5-2020
Location: Member Is Offline
Member Is Offline
|
|
We are derailing this thread, in part this is my fault.
Even if this would be viable it would be the most expensive way of obtaining absolute ethanol 
Wikipedia mentions this:
| Quote: |
"Een slurry van droogijs met een geschikt organisch oplosmiddel (aceton, isopropanol of di-ethylether) kan temperaturen tot −100°C bereiken."
|
| Quote: |
"A dry ice slurry with a suitable organic solvent (acetone, isopropanol or diethyl ether) can reach temperatures down to −100 ° C."
|
Also: https://wolfweb.unr.edu/homepage/wchalifoux/PDFs/cooling_bat...
Lists diethyl ether + CO2 @ -100 ° C.
This is not IPA or Acetone as I mentioned earlier, my apologies.
[Edited on 29-5-2020 by Belowzero]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Sorry for derailing more but I can already imagine the poor soul putting some sort of pyrophoric substance, think t-BuLi, into their reaction mixture
but spilling a single drop near that ether ice bath.
Ether cooling baths sound like an incredibly stupid idea.
- 100 is more commonly reached with liquid nitrogen in alcohol baths (MeOH, EtOH) or acetone baths.
Also this - 78 C reported all too often is bull most of the time, measure that internal temp, only rarely is it really - 78 C inside with dry ice
cooling baths. Add to that exothermic effects and the literature - 78C is really more like - 60C internal.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Actually its ok to not do face reveals. Nurdrage and chemplayer never did that.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
This link goes into detail on how to make it drinkable. I've also looked into this and came up with cryogenic freezing to seperate methanol and other
crap out of the ethanol. Whether or not it works I don't know but the link below does it perfectly
and succeeds in getting drinkable product from metho.
https://forums.whirlpool.net.au/archive/2235624
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
Cyrogenic freezing sounds like very expensive and difficult to do.
The thread linked contains absolute gibberish. First, methanol is not produced in homebrew other than trace quantities, and acetone is essentially non
toxic and it is excempted in many countries legislation actually. Homebrew has never killed anyone due to poisoning, the other additives or residues
have. All methanol poisonings are caused by (un)intentional selling of meth as alcohol. Homedistiller contains chemical analyses from batches and
methanol is one of the smallest residues.
Much easier method is to use long boka distilling column. Alcohols are inert, so steel column can be used. With 1000mm packing one can easily reflux
off methanol, and with 2000mm the separation is much more effective. You can stack 3-4 ordinary reflux columns if you dont or cant have a boka and put
a distilling head on it to do a controlled reflux. I've discussed about this in this topic:
http://www.sciencemadness.org/talk/viewthread.php?tid=155286
What you do is to reflux at 10 to 20 for 1 ratio, and separate the distillate into batches of 1-5dL in size depending on your total volume. When
distilling stripped homebrew, the separation is very clear and the distillate sits at different temp ranges when starting when the foreshots and heads
come over. Eventually it will settle to 78.2 multiplied by your barometric number (for ex 78.2*[1.020]mbar). You will need a decent thermoprobe to
monitor the condensate temp.
MEK should go off with NaOH. De Benzoate has already high BP and mixing HCl will only cause it to come over with the distillate.
For homebrew, the price of about 3-5$ per L of EtOH is pretty accurate. This includes ingredients and energy for distillation, excluding equipment
capital investment. Chemical reagent ethanol has lower requirements for purity, and when I do fractioning runs at 95%, I usually collect only about
30-40% total distillate for drinking, and the rest goes to recycling can that tastes too bad (heads and tails). Foreshots, aka the first 1-2dL I just
discard.
[Edited on 30-5-2020 by Refinery]
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Small amounts of ethanol can be purified via ester formation, purifying the ester, then hydrolyzing back to alcohol. This is overkill, though, and
requires anhydrous conditions.
I usually purify alcohol by fractional distillation with 400 mm packed column (ceramic Rashig rings), stirring with activated carbon & filtering
through Celite pad, then further purify by refluxing with KOH.
Even more rigorous purification involves adding small amount of conc. H2SO4 followed by small amount of sat. aq. KMnO4. The alcohol must be cold
(below room temp.) and not on bright daylight, otherwise KMnO4 decomposes too quickly.
It is possible to assay quality of alcohol exactly by how quickly a standardised KMnO4 decomposes to MnO2 - the longer - the higher quality alcohol.
More info in this great book:
Armarego, Wilfred LF. Purification of laboratory chemicals. Butterworth-Heinemann, 2017.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Refinery I don't think you can call a successful set of experiments and a writeup
telling others how to get the same result jibberish. Its no difference from what we do here.
|
|
|
EliasExperiments
Hazard to Self
 
Posts: 57
Registered: 3-3-2020
Member Is Offline
|
|
Wow nimgoldman that sounds very interesting. I guess the KMnO4 can oxidise the isopropanol easier then ethanol? Thank you for the post!
|
|
|
Mush
National Hazard
   
Posts: 633
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
Methanol and ethanol can be purified via their alcoholate with calcium chloride or magnesium chloride.
PREPARATION OF ABSOLUTE ALCOHOL WITH CALCIUM CHLORIDE AND LIME
J. Am. Chem. Soc. 1923, 45, 3, 857–862
Publication Date:March 1, 1923
https://doi.org/10.1021/ja01656a048
2. On concentration of such a solution a solid alcoholate, not a hydrate, begins to separate when the boiling point reaches 95-100" and there is an
equilibrium between the alcoholate and hydrate present. A quite high temperature is required to expel the alcohol from the solid alcoholate.
3. The hydrate of calcium chloride containing 4.5 moles of water (80 parts) for 1 mole of calcium chloride (111 parts) boils at 140" and from such a
solution the alcohol may be distilled completely with a strength of 90% or greater. The solution of calcium chloride of' this composition is liquid
at 140 " but solidifies on cooling.
Energy Procedia 91 ( 2016 ) 161 – 171
Reaction of calcium chloride and magnesium chloride and their mixed salts with ethanol for thermal energy storage
Formation of Ca+ (EtOH)(m) from alcohol solutions of CaCl2
The Journal of Physical Chemistry A 104(6):1079-1084
Attachment: 1-s2.0-S1876610216302922-main.pdf (770kB)
This file has been downloaded 400 times
Attachment: kohno2000.pdf (213kB)
This file has been downloaded 366 times
Attachment: noyes1923.pdf (432kB)
This file has been downloaded 350 times
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Try diluting some of your purified ethanol down to 40% and see if it goes cloudy. If it does it's possibly still got cyclohexane/ene in it. If u read
all of the linked article:
https://forums.whirlpool.net.au/archive/2235624
It goes into detail about an impurity that remained after mek and denatoniumbenzoate was removed. it uses olive oil to remove the cloudyness from the
diluted to 40% purified product.
If u r still thinking there's isopropyl in it add some kmno4 to turn it to acetone then add hydroxide to polymerize and neutralise any other crap it
formed then redistill
[Edited on 8-6-2020 by draculic acid69]
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
http://libgen.is/book/index.php?md5=6095DB33A8D84624B1CC4EE2...
/CJ
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Just to note - producing pure ethanol from vodka or sugar wash is a different story than recycling used ethanol solvent (especially from from organic
syntheses).
For example, I struggled with ethanol smelling after nail polish remover even after several distillations. Then realized some ethyl acetate formed
that just distills over with alcohol (forms lover boiling azeotrope).
Apart from esters, there can be carbonyl compounds (aldehydes, ketones), amines, acids and bases with low boiling points (e.g. HCl, NH3).
Depending on the impurity profile and intended use, one should treat the alcohol accordingly.
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
The car ethanol I have has an evaporation residue that is sticky, probably consisting some sort of detergents. After distillation with NaOH, it was
noticeably more neutral in odor, and no residue were left after evaporation and measured 95% ABV, which apparently was the starting strength because
only a simple distillation was performed.
I was wondering if I could use my SS boka still to strip it clean a lot faster than with glassware? The still can strip 6-8 liters at full rate
without reflux, so a good stock of high grade ethanol could be made with one, quick run.
What I'm worried is that could it contaminate any food grade alcohol that is later distilled? I'd remove the ceramic column packing because the
feedstock is already at 95% so it could be pushed through with full rate. Denaturing agent is MEK, aside of detergents for car use, and they'll be
reacted off with addition of few % of NaOH to the ethanol.
On the side, I have few bottles of feints that were left from potable alcohol distillations. They have 91-95% abv, but they contain impurities from
heads and tails which were put aside due to off-taste. Could they be used in chemistry as solvents?
[Edited on 16-6-2020 by Refinery]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Could MEK as a ketone be removed with bisulfite adduct? If there is 2% of it or 20 grams per liter, equimolar or slight excess of bisulfite should at
least in theory react with it. Other question is, does it precipitate or can it withstand boiling off the ethanol, not to dryness of course?
|
|
|
Whathappensif
Hazard to Self
 
Posts: 53
Registered: 9-7-2020
Member Is Offline
|
|
What about using an adsorbent material, on the basis that unless they use methanol as a denaturant, ethanol is the smallest molecule and would adsorb
fastest.
For example:
1. Fill a column with activated carbon beads
2. Flush denatured alcohol through
3. Remove beads, and evaporate + condense alcohol
If you repeat this process a few times it should concentrate the smaller molecules.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I have to add something.
Here, I have cheap MEK and denatonium benzoate denatured EtOH available(Germany, but you need to choose the right brand, this was AHK Spiritus).
I distill that twice over NaOH usually.
Once, I thought to myself, that it smells clean like wodka, and I tasted some off the tip of my finger.
It tasted like VERY strong wodka, and I thought "the early chemists tasted really worse things, lets check it!".
And so I did.
Diluted with coke it tasted just like wodka 
So if you have the right clean brand, MEK is easily removed with the self-aldol condensation.
You need to distill carefully though.
|
|
|
| Pages:
1
2 |