Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
Lead distillation
anyone have directions of what company around europe might be able to help with building an apparatus for distilling maybe as much as 100 tonnes of
lead? would be around 10m3
it would have to withstand around 1750*C, im thinking short distillation path, but due to 1750*C steel is out of the question
i think fixing something like that up all by myself is out of the question
im thinking the people who produce equipment for melting steel might be able to offer something around this type of equipment, but i dont know any
brand names of such around europe
as for heating elements, 1750*C seems to be the outer limits, so barely even possible, i imagine it would be very difficult as the lead would be all
over the vessel which could easily shortcut the heating elements, is distillation of lead ever actually done?
|
|
|
Fulmen
International Hazard
    
Posts: 1720
Registered: 24-9-2005
Member Is Online
Mood: Bored
|
|
Not to rain on your parade, but have you thought this through? 100 tons? Is that per year, or in total? And what's your budget? A few thousand €
wont buy you more than a meeting or two.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
wg48temp9
National Hazard
   
Posts: 785
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Wow why do you want to distil about 10 cubic meters of lead? Does it have something valuable in it you want to extract?
You could heat the lead by induction using a coil outside of the furnace. Molten metals at that temperature tends to be very corrosive. I
guess the crucible would have to be made of mostly of MgO or Al2O3.
Perhaps you could use a high temperature a kiln and fit with a ceramic flask the neck of which would have to protrudes from the kiln.
I melted lead in a small crucible using using the current from a high current transformers I guess that could be used or arc melting.
At ten litres in each distillation its 1,000 runs. Perhaps something bigger like the following
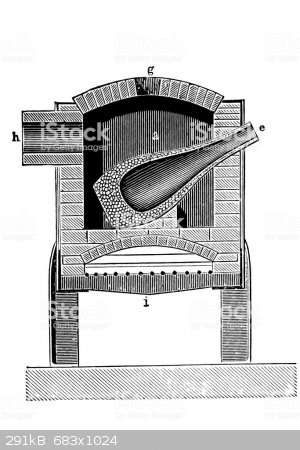
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
j_sum1
Administrator
       
Posts: 6328
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
You have two fundamental problems. One is scale. The other is temperature. Before going too far into this quest, consider your objective. There have
to be simpler processes.
For something of this scale and temp, you land right in the territory of refractory bricks such as those used in steelmaking furnaces. These are
skillfully shaped and mechanically interlock without mortar. Good luck.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Better oxidize the lead into solution and reduce it back into metal on a electrode. Cheaper and easier.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
I think Tsjerk is right. My younger brother managed to boil lead when he used to make little soldier figurines. Lead vapors are not to be trifled
with!
|
|
|
Vosoryx
Hazard to Others
  
Posts: 282
Registered: 18-6-2017
Location: British Columbia, Canada
Member Is Offline
Mood: Serial Apple Enjoyer
|
|
I see no reason why an apparatus couldn't be built by the amatuer for a few thousand dollars... I can't see the appeal but here's my thoughts...
Refractory brick and refractory cement can withstand that temperature without issue. Building a simple airtight retort from it shouldn't be hard or
even expensive, in the grand scheme of things. I've built plenty of forges/foundries, it takes some skill to build with refractory compounds but not
much. The bricks do radiate heat so I'd wrap it with a copious amounts of kaowool. Forced air propane can get up to just under 2k c. No issue there,
though manufacturing the burners would be a pain if you don't have a metal shop. I've done it but. Still hard.
Unless the lead is grossly contaminated, requiring emptying of the boiling chamber, I'd figure out some way of adding more lead to it while it's going
so as to save time with heating and cooling. Thick, capped steel tube though which you can pour more lead as it progresses.
And I'm assuming lead is the lowest boiling compound. If there are others with a similar boiling point you'd have to be really careful that none of it
came over. Cleaning of the apparatus would have to be done with a propane torch. Get used to wearing a facemask. Any water in the apparatus could
prove fatal.
But, again, I have to ask. Why. Seems like a lot of work and if you're putting that much money in to separate something (silver? Antimony?) From the
lead I'd reckon an electrochemical route would be the way to go. Is this just a thought experiment or do you have 100 tonnes of lead stashed in the
basement that the misses is nagging you about dealing with?
"Open your mind son, before someone opens it for you." - Dr. Walter Bishop
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Even with an electrochemical method, say electroplating the lead from an electrode to another and assuming 100% efficiency you would still need A LOT
of current for a long time to process 100 tonnes. 100 tonnes of lead: that's 483 kmoles. Per Faraday's law of electrolysis, you would need 2*483'000
[mol]*96485 [C/mol] = 93.2 gigacoulombs of charge transfered to electroplate all the lead. That's 93.2 billion amp*second. Assuming you can find a
power supply that can deliver (say) 1000 Amps DC, you would need just shy of 3 years of continuous operation to accomplish your goal. Not completely
out of reach, but pretty difficult.
Anyway I'm dying to find out why you want to distill 100 tonnes of lead, please provide some details!
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
the idea is seperation of elements, ontop of that the lead has near zero radiation which - despite having been unable to find anything on it, ive been
told is valuable in itself, i just feel like chemical seperation would be a mess. ontop of that electrolysis is as pointed out something that takes
quite some time
ive thought about just basic refractory bricks, but when youre dealing with one lead bar thats 100kg- just putting one of those down on those little
fragile bars, youre gonna break the whole setup with first bar just carefully lowering them down
about the temperature required- 1750*C, tungsten appears to be used for 2500*C heating, but tungsten oxidizes very easily without an inert atmosphere,
with an open vessel i think reaching a high temperature could be an issue, maybe bottom could be made of a metal with a high melting point and then it
could be heated from underneath?
the lead could be added as a liquid, this would take care of the impact issue
the reason i want to distill 100 tonnes of lead is purely monetary, without trying to be mysterious i cant go into detail, but fractional distillation
seems to be the ideal way, the metals have quite some difference in boiling point.
making the vessel would need to be quite well sealed up, my own personal dealing with lead have found it that even tiny little cracks would leak lead
i dont fear the lead fumes much, should settle out quite easily, the retort might clog up however, but this shouldnt be much of an issue with
something as soft and low melting as lead
if combustion was to be used i think lead fumes would be a more important issue as the heat would carry further, im just thinking of direct heating
here.
ive thought about writing a university as they would typically know big companies for these odd exotic chemical operations, money shouldnt be an issue
if we could just pull a bit of this material through.
|
|
|
Steam
Hazard to Others
  
Posts: 238
Registered: 25-3-2014
Location: Minnesota
Member Is Offline
Mood: Triple Point
|
|
I would also strongly encourage doing this through an electrorefining method such as electrowinning. Perhaps something like the following:
https://www.google.com/url?sa=t&source=web&rct=j&...
Designing large scale, High temperature reaction vessels for neurotoxic elements is honestly not something to be casually done. I have two degrees in
this field and am a nationally certified engineer and even I would not touch this project unless it was absolutely necessary and the client had the
budget to do it right. While your own health may be a risk you are willing to take (which I completely understand because I am the same way), it is
the risk to the people around you that is the larger problem. By distilling in a DIY lead still you would almost certainly be violating federal air
quality control acts as well as opening yourself up to a very hefty lawsuit by your neighbors (think Flint MI water) which could present you with
potentially millions of dollars of exposure per person depending on the severity of exposure. I would highly recommend, if you must do this, to use
an electrochemical method. If you run the cell at night you will save on power and you will be able to recover any precious metals in your lead as
well!
DISCLAIMER: The information in this post is provided for general informational purposes only and may not reflect the current law in your jurisdiction.
No information contained in this post should be construed as legal advice from the individual author, nor is it intended to be a substitute for legal
counsel on any subject matter. No reader of this post should act or refrain from acting on the basis of any information included in, or accessible
through, this post without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from a lawyer
licensed in the recipient’s state, country or other appropriate licensing jurisdiction.
|
|
|
unionised
International Hazard
    
Posts: 5127
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
No it hasn't
There are natural radioisotopes of lead.
https://en.wikipedia.org/wiki/Isotopes_of_lead
Zone refining seems like a much easier job (and would produce a higher purity product than simple distillation.)
https://en.wikipedia.org/wiki/Zone_melting
|
|
|
j_sum1
Administrator
       
Posts: 6328
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Ooh. Zone refining. Now there's something I have not heard of i. A while.
Excellent suggestion.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Only if the lead is very old, so that unstable isotopes of lead have had time to decay.
Lead from ancient times (eg cargo from an old sunken roman boat) is valued by physicist as shielding for certain experiments. Its a very specific
market though. Not sure you can make a lot of money with this.
| Quote: |
ive thought about writing a university as they would typically know big companies for these odd exotic chemical operations.
|
I doubt it, most universities are research institutions. Very few of them will have experience with industrial scale metal purifications. I'd rather
call a smelter or a mining company.
Purifying lead isn't very exotic. Just the method that you've chosen.
Is the metal that the lead is alloyed with very exotic?
[Edited on 24-2-2020 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I really hope these metals are used for the best; scientific equipment asking for radiation free steel.
Metals refined before atmospheric nuclear tests are precious, but I'm afraid the ships salvaged are just going for the normal metal prices...
|
|
|
unionised
International Hazard
    
Posts: 5127
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It's perfectly possible to make low-background steel today.
https://en.wikipedia.org/wiki/Direct_reduced_iron
In general you don't want air getting to your steel while you smelt it. The oxygen in air is exactly what you are trying to remove.
Use of hydrogen allows removal of practically any radioactive material from the reductant.
Iron ore isn't likely to contain significant amounts of uranium etc. (I'd guess a few ppm)
I suspect a pre-treatment with carbonate could strip most of any uranium that is present.
You could ensure that the reduction process was performed under conditions where uranium wasn't converted to metal.
|
|
|
Vosoryx
Hazard to Others
  
Posts: 282
Registered: 18-6-2017
Location: British Columbia, Canada
Member Is Offline
Mood: Serial Apple Enjoyer
|
|
Alright. I hate to be "that guy" who goes on an amateur chemistry thread and says that the method at hand is impractical and expensive, a good many
threads have been derailed by assess who can't think of anything better to say than "this isn't economical".
However.
If distilling lead by the 100 tonne quantity, in order to produce low background radiation lead metal is your goal, this isn't economical.
So much seems to be a secret... If you can't tell us what other metals are in the lead, for fear of us copying the idea, it might be more practical to
call up an industrial smelter or professional metallurgist and pitch to them. I think the reason we're bouncing around from one idea to the next is a
lack of knowledge about your situation.
"Open your mind son, before someone opens it for you." - Dr. Walter Bishop
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by unionised  | It's perfectly possible to make low-background steel today.
https://en.wikipedia.org/wiki/Direct_reduced_iron
In general you don't want air getting to your steel while you smelt it. The oxygen in air is exactly what you are trying to remove.
Use of hydrogen allows removal of practically any radioactive material from the reductant.
Iron ore isn't likely to contain significant amounts of uranium etc. (I'd guess a few ppm)
I suspect a pre-treatment with carbonate could strip most of any uranium that is present.
You could ensure that the reduction process was performed under conditions where uranium wasn't converted to metal. |
Yes, you absolutely right, but melting the iron already refined is a lot easier than refining new iron.
|
|
|
Fulmen
International Hazard
    
Posts: 1720
Registered: 24-9-2005
Member Is Online
Mood: Bored
|
|
Old lead can indeed be valuable. Not only for shielding, IIRC it's also desirable for microchip mfg. But it doesn't have to be from ancient times, a
few hundred years should be enough.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Wouldn't vacuum distillation be easier? It would lower the temperature requirements considerably.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Smelters yes. But I'm certain you can find a company that recycles lead from car batteries.
I'd go to them first with my questions.
They should be easy to find: follow the paper trail.
These companies always attract controversy and should be in the papers...
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
Steam: thanks for the heads up, this is one of the reasons i wouldnt fancy handling 100 tonnes of lead in my kitchen, let alone to just handle a
single of those bars it would be ideal to put up lifting equipment and worse
unionised:
i cant remember if lab confirmed that the lead was very low in radiation or if we simply assume so because it is infact ancient lead.
zone refining sounds doable and very easy- except the lower melting portion is in very small quantities, i dont think it would coalesce, unless you
were to grind the whole deal into granules or maybe dust, i would assume you would need to grind that up in very cold temperatures for the lead to not
be all sorts of sticky and actually turn into dust - supposing it would be possible or even make a difference in zone refining if it was as powder
would 2 molten metals seperate themselves by gravity if kept molten maybe?
vosoryx:
the low radiation lead is a bonus, i suspected the market for such to be very low but who knows
DavidJR:
vacuum distillation is an idea, would require a very large vacuum device i suppose, do you know by chance if its possible to lower boiling point
equally to that of water? i recall NurdRage managed to reduce boiling point of water to just around 20*C with a cheaply homemade setup
alright so i guess i could try hearing some smelters out who their equipment supplier is and then talk to them and hear if they have ever heard of
equipment capable of doing such or maybe know engineers that could help with this project
if car battery recyclers dont give one shit already then i might shoot them a letter haha, by chance ive seen nearby scrapyard having tonnes of car
batteries lying around, theyre dealing not just with lead melting but also some amounts of lead salts, but then again they probably operate just
around melting point of lead
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Antiswat  |
DavidJR:
vacuum distillation is an idea, would require a very large vacuum device i suppose, do you know by chance if its possible to lower boiling point
equally to that of water? i recall NurdRage managed to reduce boiling point of water to just around 20*C with a cheaply homemade setup
|
A simple water jet pump will pull a vacuum which will make water boil as low as the temperature of the water used in the pump.
For vacuum distillation of metals: see this.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
so it takes about 300*C off, of what i understand, but more interestingly- lead distillation has been done. now i just need to figure out who has done
it
0:50 they show normal BP vs vacuum BP of metals, magnesium having lower boiling point under vacuum than its melting point? so melting point should
also be shifted with vacuum. in the video it says lead can distill at 957*C - this means the whole setup could be made with steel, corrosion is lesser
issue.
even aluminium is doable in steel construction, the link you shared mentions producing metal powders by controlling condensation
this is getting off topic but this has quite some potential. except it seems steel melts at lower temperature under vacuum.
|
|
|
unionised
International Hazard
    
Posts: 5127
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The effect of pressure on melting points is tiny. You can pretty much ignore it.
You also need to remember that molten lead is a good solvent for some metals; I think lead in steel is more or less OK.
|
|
|
Texium
|
Thread Moved
29-11-2023 at 13:23 |