B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
drying isopropyl nitrite with CaCl2
Hey there,
I'm planning to make some isopropyl nitrite in order to later make sodium azide.
I have sodium nitrite salt(which I made from charcoal and sodium nitrate), isopropyl alcohol(EP) and sulfuric acid.
I more or less going to follow this preparation by "AllChemystery" - https://www.youtube.com/watch?v=RpMENDo8KRo.
Because my nitrite might have some traces of nitrate, I will avoid using HCl or tap water to prevent aqua regia from forming.
Anyway( ), I don't have any magnesium sulfate in hand(for drying), so I'm planning
to first use sodium bicarbonate to neutralize any leftover acids, followed by sodium chloride and then calcium chloride. My fear is that the calcium
chloride might react to create calcium nitrite or some other byproduct. ), I don't have any magnesium sulfate in hand(for drying), so I'm planning
to first use sodium bicarbonate to neutralize any leftover acids, followed by sodium chloride and then calcium chloride. My fear is that the calcium
chloride might react to create calcium nitrite or some other byproduct.
I know sodium chloride is "safe" to use(for drying nitrites), but I'm not sure if that's also the case with the calcium salt(I know that
sodium-chlorine bond is particularly strong ionic bond).
I searched the web and checked a few drying agents charts from the literature, but found nothing(nobody care about alkyl nitrites).
P.S. I also have some potassium carbonate.
Thanks, Ben.
[Edited on 5-2-2020 by B.D.E]
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
chemx01 used K2CO3 which you already have https://www.youtube.com/watch?v=BQLg4ijSNfc
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Thanks man, I'll try it.
The problem now I failed making the isopropyl nitrite. I think the problem is with my sodium nitrite salt. I'll try a few thing and if they all fail,
I would open another thread :S
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
NaNO2 is a food additive, especially products from meat like sausages, ham etc. It binds to hemoglobin and myoglobin so the color of the meat product
looks more fresh with it than without it. Perhaps you can buy it that way, but be careful, it could be a mixture of e.g. 99% NaCl + 1% NaNO2 then do
not buy such a crap.
NaNO2 is usually made by heating NaNO3 + Pb, but Pb and its oxides/salts are quite toxic. Perhaps reacting with C instead, but then harder to drive
the reaction (similar to black powder, just missing S).
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
This is sort of what I did. I've mixed 2eq of NaNO3 with 1eq of charcoal, grinded them together to an homogeneous mixture which I then
lighted on fire with some birthday sparklers.

The reaction I hoped for was this:
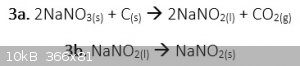
But a lot(if not all) of my product has decomposed into NaO2 which later reacted with the water I've added, to make NaOH(as evident by the
pH paper turning blue).
I actually had a chance to buy some extra pure NaNO2 a couple of days ago, but I thought to myself "nahh I have NaNO3, no problems".
Anyway, at this point it has become kind of a challenge for me. I feel like I should be able to do it 
[Edited on 6-2-2020 by B.D.E]
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Instead of the fast reaction ("depleted black powder lacking S"), couldn't it be done in some "controlled way" by heating NaNO3, when it melts, turn
the flame off and insert C gradually in small portions like pieces of charcoal using e.g. long pliers? Just an idea, I won't try it as I have enough
of NaNO2.
Also Fe could be used instead of Pb for NaNO2 production, but how to separate ferrate from the product... yes using Ba but again toxicity...
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Just use Pb instead of reinventing the wheel. The preparation of nitrite has been tested to death. Lead works fine(ish). The waste is not that bad, at
least none of the salts are soluble.
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
I did this decades ago using NaNO3 ( bought as a fertilizer). There was a little workup involved after but it was a success.
Currently I only have perhaps a kilogram of it but it was bought cheaply online.
Make it if you must, but be aware that it is available online.
I actually have probably 500+ kilograms of nitrate salts in my barn covered with a tarp. This is mostly Ammonia Nitrate with 50 to 75 kg being the
sodium.
I was raised on a farm.
[Edited on 2/7/2020 by morganbw]
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Thanks everyone,
I'm not trying to invent the wheel, I just don't have any lead in hand...
I tried bubbling NOx into KOH solution(with only traces amounts of air at the beginning of the reaction), but the yield was terrible..
I took a little break from this because I decided to just buy it, but it turns out my local supplier doesn't have any nitrite in stock. Also, I'm
beginning to think that maybe the nitrate does interfere with the reaction(of making isopropyl nitrite) somehow.. but that just a thought.
Anyway, I've made some KNO3. Those are my new "plans":
1. To follow THIS preparation, only with copper instead of leads. I have some CuSO4 on, so I can reduce it to a fine powder and mix it with the nitrate.
2. To carefully heat the KNO3 to 400C for a long period of time. KNO3 melts at 334C and boil/decompose at 400C. KNO2 however melts and decompose at
440C.
I'll update if anything would work.
|
|
|