| Pages:
1
2 |
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
3 NaClO → 2 NaCl + NaClO3 > KCl + NaClO3 → NaCl + KClO3
Boiling bleach disproportionates sodium hypochlorite into sodium chloride and sodium chlorate...
They say the boiling is required for this reaction to work; you can't simply let the bleach evaporate.
Question is how much boiling time does it take for this disproportion to take place ?
I would rather just boil for a few minutes in the microwave (I can bring the microwave outside) if that's all it takes then set the solution aside to
evaporate and do the next step in a few days.
I like this website https://www.thoughtco.com/potassium-chlorate-bleach-and-salt...
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
The disproportionation happens faster at higher temperatures, it is not the process of boiling it, but the elevated temperatures that are necessary.
You need to keep it at high temp for long enough that the reaction has time to "complete", but I don't know how long would be a good choice. I would
guess that 30 minutes is ok, but longer will evaporate some of the water, so your yield will be higher when you add the potassium salt.
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
I am going to skip the microwave and use the side burner on the BBQ. If the wind ever quits.
But faster at higher temperatures, very interesting because at "a pressure of 1 bar or approximately 100 kPa (15 psi) above atmospheric pressure,
water will reach a temperature of 121 °C (250 °F).
Maybe that would increase yield past whats obtained from boiling at what ever temp bleach normally boils.
Might have to explore that.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
A test would be if the solution reacts with hydrogen peroxide given there is no transition metal contaminates so if oxygen forms bleach is still not
fully converted
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
As a rule of thumb, a 10 degree (kelvin) rise in temperature will double the reaction rate, so 120C should be about 4 times the rate at boiling. Note
that it won't affect the yield directly, just the time it takes to get to completion. The advantage of boiling is that by reducing the volume of the
solution, less product will be dissolved in it rather than filtered off as crystals, so your yield will be higher.
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
I finally did this project.
I used a $17 3 quart slow cooker and simmered the bleach for a day for then took the lid off and it took about 10 hours to evaporate down to where I
saw the sodium chloride collecting on the bottom and a crust on the top. Stirring the stuff on top seemed to redissolve the top crust into the liquid.
Did that a few times over the next hour till it seemed it just sank to the bottom.
With the lid off the evaporation drops temperature quite a bit. The heating element doesn't produce enough heat to boil it with the lid off.
Filtering. The remaining liquid kept dissolving the coffee filter and it would break then I doubled them up and got it filtered.
I didn't measure it was about 500 ml of liquid. Then I heated an equal amount of water and dissolved the potassium chloride salt substitute,
filtered , reheated and added it to the first solution and right away I saw some precipitation starting. Then ice bath cooling and it began
collecting on the bottom.
Used about 68 grams of potassium chloride as I measured 20 left from an 88 g container.
And just measured 60 grams of final product minus 1 or 2 I used for tests.
Was very sloppy with measuring and recording, I just wanted to see if this would work and it does.
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by symboom  | | A test would be if the solution reacts with hydrogen peroxide given there is no transition metal contaminates so if oxygen forms bleach is still not
fully converted |
I am getting some peroxide before the next batch.
Even though your never supposed to smell chemistry projects it didn't smell much like bleach after the simmering but like I wrote above it did seem to
dissolve the coffee filter suspended by the edges and cause it to break by the weight of the liquid. Maybe that was un reacted bleach causing that.
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
This time simmered it for a whole day then another day evaporating with the cover off till the crystals formed and when filtering the single filter
did not get eaten and rip in the process.
This time I am cooling the filtrate and something is precipitating out. I think its sodium chloride maybe some sodium clorate too I don't know but I
am going to filter that stuff out and then when I get more potassium chloride for the next step see what happens.
And peroxide for that test too.
I need to order a microscope. Probably learn a lot looking at all these crystal things with a microscope. I know regular salt is all cubes.
[Edited on 11-3-2020 by Pyro_cat]
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
I never ordered the microscope. But I am going to.
Think I am on my 6th batch now, the hypochlorite for swimming pools works better then bleach but there is this brown yuck that makes it through the
filter.
Its interesting the slow gravity filtering the potassium chloride it floats on top of the boiled bleach and makes this disk of chlorate between the
layers then you shake it and starts to precipitate even before the freezer.
Its also interesting watching the NaCl crystals form a top layer as soon as you take the top off the crock pot once the bleach disproportionates.
Are all those crystals NaCl or are some sodium chlorate if you boiled it down to far? and yield loss.
I am going to have to dry that whole mess out mix some with sugar and see what happens. See of it burns.
What I also noticed on this last batch is I was adding the potassium chloride to a soda bottle full of hot water and it cooled it.
For a second I thought I should have used hotter water but I did use hot water and it felt not chilled but colder then ambient so it must be doing
that instant cold pack endothermic thing.
[Edited on 6-4-2021 by Pyro_cat]
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
Drying out the Kclo3 and its alot like the getting the water out of Epson's salt (magnesium sulfate heptahydrate) experiment. Its like that same
slush that just refuses to dry out.
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
I think it would be better to start with Ca(ClO)2. At least, this compound can be obtained in the free market.
Women are more perilous sometimes, than any hi explosive.
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
I saw it in the pool section "73% Calcium Hypochlorite"
Online I just read another brand "68% Calcium Hypochlorite, which means 67.4% free chlorine"
Means the rest is calcium and oxygen or some filler junk for more bulk?
MSDS time
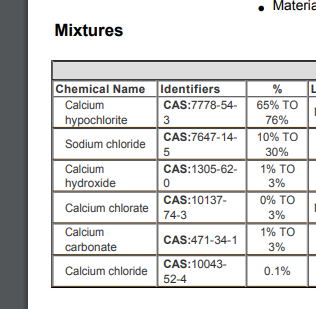
All that NaCl but Ill try it.
|
|
|
KCLcoal
Harmless

Posts: 7
Registered: 30-8-2019
Member Is Offline
|
|
Quote: Originally posted by Pyro_cat  | Boiling bleach disproportionates sodium hypochlorite into sodium chloride and sodium chlorate...
They say the boiling is required for this reaction to work; you can't simply let the bleach evaporate.
Question is how much boiling time does it take for this disproportion to take place ?
I would rather just boil for a few minutes in the microwave (I can bring the microwave outside) if that's all it takes then set the solution aside to
evaporate and do the next step in a few days.
I like this website https://www.thoughtco.com/potassium-chlorate-bleach-and-salt... |
Sodium hypochlorite decomposes into sodium chloride and oxygen molecules when boiled (100 ° C). On the other hand, when heated at a temperature of 80
° C for a long time, a disproportionation reaction that produces chlorate ions mainly occurs.
For amateurs, the boiling method is recommended for the production of chlorate as a method of removing the unstable salt hypochlorite ion from the
chlorate. (Boil time about 10 minutes)
For your purposes, boiling will cause only some disproportionation reactions and most of the hypochlorite ions will only decompose.
On the other hand, at a temperature of about 80 ° C, the target disproportionation reaction becomes the mainstream, and the decomposition reaction
into sodium chloride and oxygen molecules can be suppressed. However, it requires long-term heating.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Online
Mood: No Mood
|
|
Does anyone have a paper that gives a clue as to the rate of disproportionation at different temperatures and at different concentrations of excess
NaOH?
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by KCLcoal  |
On the other hand, at a temperature of about 80 ° C, the target disproportionation reaction becomes the mainstream, and the decomposition reaction
into sodium chloride and oxygen molecules can be suppressed. However, it requires long-term heating. |
Thanks
I don't have any bleach so I just put water in the crock pot and set it to low see what temp it gets to. A crock pot is ideal for long term heating,
done several batches in it and the ceramic is not effected at all.
I will check the temp in a few hours. Its a little windy so it will be lower then when its still out. On high with any breeze it would not boil
unless covered with a towel.
Could also use the variac to get that target temp on high setting too.
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
Its at 77C on the low setting. Nice.
|
|
|
BauArf56
Hazard to Self
 
Posts: 68
Registered: 22-8-2019
Location: between the moon and the sun
Member Is Offline
Mood: energetic
|
|
bubbling chlorine through an hot sodium hydroxide solution produces sodium chloride and chlorate, which can be displaced with a potassium salt to give
insoluble potassium chlorate. This method is not very efficient, as it produces lots of chloride and hot naoh is too corrosive. This was just an idea
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
I have not yet seen a reliable and well-described method Cl2 + NaOH = NaClO3. Or Cl2 + KOH.....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
I did. Bubble chlorine gas through a boiling solution of K2CO3. So simple. One detail- the tube (its part in liquid) must be wide enough otherwise it
will be blocked with crystals of KClO3. I made such an experiment. The only thing to be kept in mind is to complete the process- to transform all
carbonate.
Women are more perilous sometimes, than any hi explosive.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
So easy? Interesting.....Equation say : K2CO3 10g + Cl2 5,1g = 3,2g CO2 + 9g KCl + 3g KClO3. Advantige is, that KCl is 88x more soluble in water than
KClO3.
Solubility.....KCl......277,70g in...100g H2O at 0 C
Solubility.....KClO3.....3,13g in...100g H2O at 0 C
Now we obtain 3x more KCl than KClO3. But KCl stay in solution and KClO3 will crystalised. Maybe with pretty good purity. Cl2 is easy produce from
pool tablete. Interesting methode.
And because KCl + CO2 during reaction again create K2CO3 which again reacting with Cl2.....all process may ended with pretty pure KClO3. Without KCl
in solution.
Thanks.... ...Very interestling ...Very interestling
[Edited on 2-5-2021 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  |
Now we obtain 3x more KCl than KClO3. But KCl stay in solution and KClO3 will crystalised. Maybe with pretty good purity. Cl2 is easy produce from
pool tablete. Interesting methode.
And because KCl + CO2 during reaction again create K2CO3 which again reacting with Cl2.....
[Edited on 2-5-2021 by Laboratory of Liptakov] |
Do not disappoint me, mate. HCl is a much, much stronger acid than H2CO3. KCl won't react with CO2 (nor H2CO3). The only advantage of this method is
that you'll have no need for KOH.
Women are more perilous sometimes, than any hi explosive.
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 188
Registered: 12-7-2018
Member Is Offline
|
|
I dont think this reaction works with Alkali carbonats. In the Book "Bleichchemikalien" they wrote. That if Soda is used it forms only hypochloric
acid.
Na2CO3+2Cl+H2O = NaCl+ NaHCO3 + HOCL
If you add more chlorine the reaction is
NaHCO3 + 2Cl = NaCl + CO2 +HOCl
I think the reaction is the same for Potash.
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Never heard about electrolysis ? This releases ClO- which will be oxidized to ClO3- when the solution is above 60 C. No toxic Cl2 or bleach needed,
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Alkoholvergiftung  | I dont think this reaction works with Alkali carbonats. In the Book "Bleichchemikalien" they wrote. That if Soda is used it forms only hypochloric
acid.
Na2CO3+2Cl+H2O = NaCl+ NaHCO3 + HOCL
If you add more chlorine the reaction is
NaHCO3 + 2Cl = NaCl + CO2 +HOCl
I think the reaction is the same for Potash.
|
I think nothing on the issue. I know. I read it in one of the books on the inorganic synthesis and I have its text (no English). And I made this
preparation myself. The solution must be hot (must boil) and the reaction goes just as I described.
Women are more perilous sometimes, than any hi explosive.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Interesting, that solution must boil. Maybe is it a key for successful. Reagents are easily available and process is relatively easy. Therefore is it
interesting. Against electro cell method....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
| Pages:
1
2 |