CycloKnight
Hazard to Others
  
Posts: 128
Registered: 4-8-2003
Member Is Offline
Mood: Still waiting for the emulsion to settle.
|
|
Allyl Bromide Preparation
Overview
Allyl alcohol was prepared from glycerol and formic acid under inert atmosphere, hydrolysed with NaOH and fractionally distilled to yield the 73 %
allyl alcohol water azeotrope. This was then reacted with 48 % hydrobromic acid and sulfuric acid and the allyl bromide distilled as per the
conventional method. It was then redistilled with 3A molecular sieves drying agent to yield the final product which is stored over additional 3A
molecular sieves. Allyl bromide I’ve prepared in this way has been used as is, without further drying nor refinement, for the preparation of
Grignard reagent and in Grignard reactions, so has been proven suitable for such application(s).
The allyl alcohol preparation is based on patent: WO2008092115A1
“Conversion of glycerol from biodiesel production to allyl alcohol”
https://patents.google.com/patent/WO2008092115A1
Allyl alcohol is an irritant and potent lachrymator, therefore suitable overhead ventilation was used.
This is effectively a rudimentary distilling exercise in inert atmosphere, with reaction temperature measurement in addition to the usual still head
temperature measurement for distilling.
Basic equipment was used as depicted in the images, whilst I used a 500 ml 3 neck flask for convenience, an ordinary 500 ml RBF could easily have
sufficed with the appropriate fittings. Reaction mixture temperature was measured with a thermocouple probe in a sealed glass tube mounted using a
thermometer adapter.

Allyl Alcohol Preparation (2 runs in total)
Reagents per run:
165 g glycerol
144 g 85 % formic acid (3 distillation cycles, 48 g per cycle)
(Therefore a combined total of 330 g glycerol, and 288 g 85 % formic acid were used in this experiment.)
Argon was used for the inert atmosphere, though only really necessary at the start before the vapour displaces the air.
1st cycle
165 g glycerol was added to the 500 ml RBF, along with 48 g 85 % formic acid. Stir bar also added.
Setup for distillation with condenser, vigreux column, addition funnel, and still head thermometer. During the distillation, reflux/air cooling from
the column helps to keep the formic acid from distilling, increasing yields by about 15 % or so compared to when I’ve used without.
Start : Air is displaced with argon. Mantle heat is set at about 65 – 75 % of maximum, and maintained at this setting. As the solution heats, the
viscosity decreases to allow the stir bar to stir, stirring was started as soon as it was possible to do so.
Target reaction temperature I’ve used is about 235 – 245 C, attained after water distils off.
T + 15 minutes: Reaction temperature = 130 C, still head temperature = 100 C, water distilling.
T + 45 minutes: Reaction temperature = 175 C, still head temperature = 100 C, water distilling.
T + 52 minutes: Reaction temperature = 216 C, still head temperature = 99 C, water distilling, approx. 15 ml collected.
T + 55 minutes: Reaction temperature = 224 C, still head temperature unstable, varying between 91 – 97 C, CO2 evolution, though no significant
frothing.
T + 68 minutes: Reaction temperature = 240 C, still head temperature at 91 C, heat setting reduced to 60 %. Approx. 25 ml collected. Product
distilling at about 1.5 drips per second.
T + 73 minutes: Reaction temperature = 240 C, still head temperature at 86 C, approximately 1.5 drips per second of distillate.
T + 90 minutes: Reaction temperature = 246 C, approximately 55 ml collected. Heat is turned off and allowed to cool down to below 200 C. Thus
concluding the first of three cycles. (The water distillate wasn’t discarded, all the distillate was kept together. Water will be needed for the
hydrolysis later.)
90 minutes per cycle.

Another 48 g of 85 % formic acid is added to the addition funnel, and then added to the reaction mixture over about 1 minute. The heating is then
turned on as before, and the cycle is repeated, and then repeated again with the remaining 48 g 85 % formic acid.
At the end of the 3rd cycle, there was just under 200 ml distillate, and about 20 ml or so of dark orange liquid remaining in the distilling flask.
Total elapsed time was about 5 hours.

The above was repeated the next day, with another 165 g glycerol, and 144 g 85 % formic acid. Total combined distillate was about 400 ml.
*No further inert atmosphere required*
Distillate hydrolysis / allyl formate decomposition
1 hr reflux with 80 g NaOH
The RBF used for the distillation was cooled, cleaned out, and the clear distillate (~400 ml) added. The RBF was placed in a water bath, and 80 g NaOH
slowly added in 3 portions.
Caution: about half of the NaOH will boil the mixture without cooling, so external cooling is essential, or a prolonged delay between additions.
The glassware was setup for reflux, and refluxed for just over 1 hr.

By the end of the reflux/hydrolysis, the solution was dark red-burgundy coloured.
The allyl alcohol – water azeotrope was then fractionally distilled, slowly, from the dark alkaline solution, collecting the fraction 87 C – 90 C.

(The pure 72.9 % azeotrope boils at 88.2 C, and has a density of 0.905 g/cc).
Distillation required about 2.5 hours, with 200 g collected. This was then redistilled, to collect the constant boiling azeotrope, this required about
1 hour (in previous runs, this re-distillation was omitted without problems, so it isn’t essential for allyl bromide preparation).
Total yield = 192 g. Density = 0.90 g/cc.
It was then transferred to an amber storage bottle, and the internal air displaced with argon to attenuate the formation of explosive peroxide(s).
No titration was carried out to determine precise purity of the allyl alcohol, however previous experience has shown that it is sufficiently pure for
the preparation of allyl bromide in the following step.

Only 100 g of the 73 % allyl alcohol was used for the following allyl bromide preparation, the remaining 92 g was kept aside for future
experiments.
Allyl Bromide Preparation
Reagents:
321 g 48 % hydrobromic acid
86 g >95 % sulfuric acid (x 2)
100 g 73 % allyl alcohol
3A molecular sieve was used as drying agent, though other drying agents could be used instead.
Glassware/equipment is setup for distillation, with addition funnel. Vigreux column was used, but probably not necessary.
With magnetic stirrer on, 321 g 48 % hydrobromic acid, 86 g sulfuric acid and 100g 73 % allyl alcohol were added to the RBF, in that order. After
they are added the solution colour was orange.
Note: sulfuric acid will readily polymerise allyl alcohol to red tar, so it’s important to get the order of addition correct!
A further 86 g sulfuric acid (purple drain unblocker) was added to the addition funnel.

With the equipment setup for distillation (including still head thermometer), with vigorous magnetic stirring the additional 86 g sulfuric acid was
slowly added over about 3 minutes. This caused the solution to heat up and distil slightly, the solution colour changed to a darker orange. After a
few more minutes when the distillation subsided, the heating was started on medium heat. Care was taken to ensure the vapour didn’t overwhelm the
condenser.
This required about 30 minutes, still head thermometer reading gradually increasing to about 65 C or so. When water starts to come over and sits on
the allyl bromide, it’s about time to stop.
(The small amount of tar that remains in the distilling flask readily dissolves in and was cleaned using white spirits.)
All of the distillate was added to a 125 ml separating funnel, and washed with 20 ml of 5 % K2CO3 solution.

After settling, the lower allyl bromide layer was drawn off and added to a dry 500 ml RBF in preparation for the final distillation.
Note: Pure allyl bromide bp is 70-71 C (ref: chemspider)
https://www.chemspider.com/Chemical-Structure.7553.html
Equipment is setup for fractional distillation (w/vigreux column) and distillation started. After the lower boiling fraction, up to around 68-69 C or
so, has come over, some water will have also and can be seen floating as droplets on the surface of the distillate. At this point the distillation was
stopped, cooled and then 2 teaspoons of 3A molecular sieve added to the RBF. Dried by allowing to stir for 15 minutes. The lower boiling fraction is
discarded, the receiver cleaned and dried.
The distillation was then continued, slowly (about 1 drip/second). Collecting the pure allyl bromide fraction.

Final yield was 111 g, density measured to be 1.4 g/cc. This was then transferred to a dry jar containing 8 g 3A molecular sieve, then placed in a
freezer for storage. No inert atmosphere has been used for storage thus far.
I’ve stored water clear allyl bromide in this way for 2 months and counting, and with no discernible discolouration nor decomposition.
Attachment: Allyl Alcohol from Glycerol Patent.pdf (124kB)
This file has been downloaded 644 times
[Edited on 11-12-2019 by CycloKnight]
|
|
|
Boffis
International Hazard
    
Posts: 1915
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Nice write up CycloKnight! I have often contemplated carrying out this preparation but was put off by my perceived risk of ending up with a flask full
of black c**p. But you seem to end up with only a small amount of dark residue. Is it water soluble?
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
epic x))))
|
|
|
Pumukli
National Hazard
   
Posts: 724
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
A very nice preparation, thanks for sharing!
I would have thought that allyl-alcohol and sulfuric acid would produce tar even if diluted by HBr but it seems this is not the case. Probably the
water in 48% HBr is enough to inhibit polymerization.
Why is substitution of the OH with Br is preferred over say HBr addition to the double bond? Water in the system?
|
|
|
Metacelsus
International Hazard
    
Posts: 2554
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by Pumukli  |
Why is substitution of the OH with Br is preferred over say HBr addition to the double bond? Water in the system? |
The mechanism for allyl bromide formation is loss of H2O from the protonated alcohol to form an allyl cation, followed by addition of the bromide
nucleophile. SN1 mechanisms are favored for allylic alcohols due to the delocalization (and thus stabilization) of the cation.
HBr addition to the double bond could possibly take place, but this would be relatively disfavored. The alkene, instead of the alcohol, would have to
be protonated, and this is much less likely to happen.
|
|
|
Fery
International Hazard
    
Posts: 1108
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
CycloKnight beautiful and thank you for sharing. Allylalcohol synthesis from glycerol and formic acid (or dehydrated oxalic acid) is something I would
like to try in the future though I have 500 ml of allyl alcohol and 1 l of allylbromide. My allylalcohol is in big sealed glass ampule and I do not
have hearth to break it. But I would like to synthesize e.g. allylpentanoate (pineapple scent not found in nature unlike natural ethylbutyrate,
ethylhexanoate).
One step interested me - using inert gas for expelling oxygen (in the patent attached they used nitrogen) - do you think it is really necessary and
does it increase yield significantly? You did it only once at the beginning, never at repeated steps. When your glassware cooled down and vapors
condensed, by my opinion some air entered you flask. If there would be 500 ml of air inside at the beginning, that is approximately 0,1 g of O2. Does
it hurt or not? Perhaps it could induce acrolein formation?
I always try to reduce unnecessary steps, but perhaps it could be wrong in this synthesis and I should follow every step carefully and use argon or
nitrogen too.
|
|
|
monolithic
Hazard to Others
  
Posts: 436
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Nice work. Plan on doing a write up on the impending Grignard reaction? 
|
|
|
CycloKnight
Hazard to Others
  
Posts: 128
Registered: 4-8-2003
Member Is Offline
Mood: Still waiting for the emulsion to settle.
|
|
Thanks fellas!
Quote: Originally posted by Boffis  | | I have often contemplated carrying out this preparation but was put off by my perceived risk of ending up with a flask full of black c**p. But you
seem to end up with only a small amount of dark residue. Is it water soluble? |
There wasn't any tar produced during the allyl alcohol distillation, but there was some tar remaining in the flask after the first allyl bromide
distillation. This was an amber coloured residue that remained after the dark solution was emptied. This tar didn't dissolve very well in acetone nor
ethyl acetate, but a small amount of white spirits dissolved it immediately, so no risk of messy flasks, etc. After the final allyl bromide
distillation, I didn't take any photos because there was nothing to see other than a clean, empty flask with 3A molecular sieve in the bottom.
I also neglected to mention in the write up that whist there was a lower fraction, there was no higher boiling fraction, so actually the final
distillation doesn't have to be run as slowly as I did.
Quote: Originally posted by Pumukli  |
I would have thought that allyl-alcohol and sulfuric acid would produce tar even if diluted by HBr but it seems this is not the case. Probably the
water in 48% HBr is enough to inhibit polymerization. |
You are right about the tar. Some allyl alcohol was degraded, I didn't take photos but by the end of the allyl bromide reaction/formation and
distillation, the solution was very dark red, and with a thin film of black tar floating on the surface.
Quote: Originally posted by Fery  | Allylalcohol synthesis from glycerol and formic acid (or dehydrated oxalic acid) is something I would like to try in the future though I have 500 ml
of allyl alcohol and 1 l of allylbromide. My allylalcohol is in big sealed glass ampule and I do not have hearth to break it. But I would like to
synthesize e.g. allylpentanoate (pineapple scent not found in nature unlike natural ethylbutyrate, ethylhexanoate).
One step interested me - using inert gas for expelling oxygen (in the patent attached they used nitrogen) - do you think it is really necessary and
does it increase yield significantly? You did it only once at the beginning, never at repeated steps. When your glassware cooled down and vapors
condensed, by my opinion some air entered you flask. If there would be 500 ml of air inside at the beginning, that is approximately 0,1 g of O2. Does
it hurt or not? Perhaps it could induce acrolein formation?
I always try to reduce unnecessary steps, but perhaps it could be wrong in this synthesis and I should follow every step carefully and use argon or
nitrogen too. |
I'm quite sure that the inert atmosphere is essential. Although I've never tried this particular reaction without argon, I have some experience of
working with hot glycerol back when I was formulating an isoeugenol synthesis. The first run I did with glycerol, with no inert atmosphere, and only
to 150 C, for several hours. It was a complete mess. The glycerol was converted to a black, treacle-like substance, resembling burnt sugar. It took a
bit of work to clean out.
For the allyl alcohol prep, I didn't take many pictures of the solution later in the reaction, but there is virtually no colour change for the first
couple distillation cycles or so. Even though the temperature is way up at 240 C, without inert atmosphere that simply wouldn't be possible.
Yep, you betcha. 
[Edited on 12-12-2019 by CycloKnight]
|
|
|
CycloKnight
Hazard to Others
  
Posts: 128
Registered: 4-8-2003
Member Is Offline
Mood: Still waiting for the emulsion to settle.
|
|
Quote: Originally posted by Fery  |
One step interested me - using inert gas for expelling oxygen (in the patent attached they used nitrogen) - do you think it is really necessary and
does it increase yield significantly? You did it only once at the beginning, never at repeated steps. When your glassware cooled down and vapors
condensed, by my opinion some air entered you flask. If there would be 500 ml of air inside at the beginning, that is approximately 0,1 g of O2.
|
I have a large argon cylinder that I also use for tig welding. The argon is fitted with a tig secondary regulator (with a flow indicator), I use a
bubble bottle (containing dry toluene) to gauge the flow, to help conserve argon. It is therefore very easy to flush out flasks with argon, and then
maintain a very subtle argon flow, of perhaps a bubble every few seconds or so.
Before starting each distillation cycle, the internal atmosphere was displaced with argon (by temporarily increasing the argon flow) so no air entered
at any time when at high temperature.
[Edited on 12-12-2019 by CycloKnight]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2913
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
That is a great writeup and experimental detail. I'd give it an A+ if I was teaching a class.
As to the stability of the allyl group in HBr, most of the time you want to hydrohalogenate a double bond, you use a dry halogenated solvent and as
concentrated of an acid as you can find. If there is an activated alcohol, like a benzyl or allyl alcohol, it will exchange first, in many cases,
but if not, the double bond can add HCl or HBr, typically in a Markovnokov manner. The reaction is in equilibrium, and can go forewards or
backwards, depending on the amount of water/acid/SM/product present, so using dehydrating conditions may favor the elimination as well. In fact HBr
should be faster than HCl to add to the double bond than HCl. But water can compete with the HBr and either convert the bromide back to the alcohol
or add back to the double bond, so the amount of water present can affect the product ratio.
But in this case, the product (allyl bromide) can distill quickly, and once it is away from the reaction, it no longer sees the super acidic
environement. If the double bond adds HBr, you would get 1,2-dibromopropane, but its BP is about 140 C, so it cannot distill easy, so it can then
dehydrohalogenate back to allyl bromide under the equilibrium. So you likely have a mixture of the two compounds, and since the allyl bromide
distills away at about 70 C, the reaction equilibrium shifts, and more is formed. That is Le Chatelier's Principle at work. At least that would be
my explaination of it.
|
|
|
Fery
International Hazard
    
Posts: 1108
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
I would like to write my own opinion.
The inert gas is probably unnecessary. Seems water steam expels air and oxygen quickly. Keeping T below 260 C seems to be more important.
I will post more when completing everything, here just a few pictures (after 6 rounds still no tar, just orange color).
Writing this with still tears in my eyes, secretion from nose and irritated throat (though I even led a hose from the apparatus output to outside of
building) :-D
Now I know why the commercial allyl alcohol I've bought recently came in a sealed ampule.
After 3 rounds:
 
After 6 rounds:
 
|
|
|
Fery
International Hazard
    
Posts: 1108
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
allyl alcohol preparation from glycerol + formic acid
Be very careful - allyl alcohol has significantly higher toxicity (pls read the pdf attached) than other alcohols !!! CO2 evolved during reaction with
some allyl alcohol vapours must be discharged outside of building using a hose. Fumehood required and if you have suitable gas mask + efficient filter
even better. It is strong lachrymator, irritating eyes, nasal mucosa, throat, airways.
Both chemicals are quite cheap and easy to buy. Glycerol is readily available for home-made cosmetics. Formic acid could be obtained certainly from a
bee-keeper shop if you can't buy it from a chem supplier.
In the first step, glycerol is esterified into monoformate, in the second step the ester loses CO2 and H2O so allyl alcohol produced.
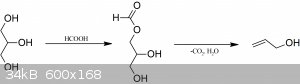
Reagents: Glycerol (anhydrous, I used pharma grade 99,5%), formic acid 85% (I used analytical grade, but technical grade should work too).
Apparatus: 2-neck 1000 ml RBF round bottom flask, 300 mm vigreux colum, 75' thermometer distillation adapter, thermometer adapter, thermometer upto
110 C, thermometer upto 300 C, 300 mm Liebig condenser, VACUUM TAKE-OFF ADAPTER (!!! THIS IS VERY NECESSARY !!!), A HOSE LONG ENOUGH FROM THE VACUUM
TAKE-OFF ADAPTER TO THE OUTSIDE OF BUILDING (!!! THIS IS VERY NECESSARY !!!), 2-neck 250 ml RBF + 1 stopper (to open the flask only when removing the
distillate, the distillate is removed by such a way that the stopper is removed, rotating the vacuum take-off adapter holding the flask, the
distillate poured from the side arm of the flask, the flask quickly stoppered again and the adapter with the flask rotated back).
Method:
To the 1000 ml RBF were placed boiling chips, 330 g of anhydrous glycerol and 100 g of 85% formic acid (the amout of acid ranged in 96-104 g it is not
critical), mixed well. A HOSE WAS CONNECTED TO THE VACUUM TAKE-OFF ADAPTER AND WAS LED TO OUTSIDE OF BUILDING.
The thermometer upto 300 C was placed in the side arm of the RFB and submersed into the liquid with its bulb close to the flask bottom so it stays
submersed even when the volume of reactants decreases due their consumption during the reaction. The thermometer upto 110 C was inserted into the 75'
thermometer distillation adapter.
The flask was heated with strong flame using gas burner. It is suggested that the first distillate (water and formic acid) distills in 15 minutes and
the T inside flask reaches 195 C in 30-45 minutes when the product (allyl alcohol) starts to distill. It is hard to estimate the intensity of heating
because it is different for the first round when all glycerol present and certainly lower flame required during the third round when a lot of glycerol
already used up. When the heating is too strong (boiling in 5 minutes and product distillation in 15 minutes) it seems to have negative impact on the
yield too, apparently less glycerol formate esterified and more of formic acid just distilled out of the reaction mixture.
The distillate coming when the T in the reaction mixture is below 195 C contains mostly water and formic acid, perhaps it is worth to recycle formic
acid from it.
When the T of reaction mixture reaches 195 C the dropping from condeser ceases temporarily and T inside reaction is increasing, at some point the
distillation resumes again at a speed of 1-2 drops per second (the product). It is hard to estimate this point just from the 110 C thermometer in the
distillation head which is unstable at this point - if the heat is too strong the CO2 coming from the reaction is too hot so the T could reach even
110 C which is undesirable (I tried that) although b.p. of allyl alcohol is 97 C and azeotrope of allyl alcohol with water b.p. 88,89 C (72,3%
alcohol). On the other side when the rate of distillation is too slow the T could indicate even less than 80 C which is caused by a condensation above
the thermometer bulb so then by cooled drops running down to the thermometer bulb. Don't rely on the thermometer in the distillation head too much,
the more important thermometer is the one submersed in reaction mixture (thermometer upto 300 C) - without this thermometer it is almost impossible to
determine the point when water stops to distill and product starts and when the formic acid is used up and the flame should be turned off (though I
tried that - no success, every round has different amount of reactants, different flame strength, some air blowing or with different temperature
around unisolated column caused by different hour of the day, etc).
So when the T of the reaction mixture reaches 195 C, remove the water+formic acid (about 45-55 ml) from the flask capturing the distillate (without
detaching the flask - just rotating the take-off vacuum adapter with the flask and using side arm of the flask to pour off the distillate) and start
to collect the allyl alcohol, when the T reaches 260 C turn the heating off and let the mixture to cool down (few drops of the product will still pass
through the condenser on cooling down the reaction mixture). About 65-105 ml of the product obtained (less product when heating was too strong and
also when the heating too week then acrolein formation with negative impact on the yield too).
When the T decreases to 100 C, add second portion of the formic acid (100 g) and repeat.
Then the third round with the same amount of formic acid and at the end there is only little of glycerol remaining in the RBF.
To the remaining yelow glycerol I added extra 292 g of fresh pure colorless glycerol and repeated the 3 rounds again. This second serie I was more
brave and applied too strong heating which seemed to have negative impact on the yield. Too slow heating has also negative impact (acrolein
formation). It is hard to reproduce all 3 rounds exactly due to different mass of reactants (some of glycerol used up every step, so the first round
requires stronger heating than the third one).
It is necessary to adjust heating during every step, initial heating is stronger and when reaching desired temperatures it is decreased to approx 1/2,
sometimes 1/3 and rarely 1/4 (if the initial heating was too strong) - just watch thermometers, especially the one sumbersed in reaction mixture
(allyl alcohol collected when reaction mixture temperature in range 195-260 C from which at both extremes only very little of distillate and most of
distillate at 225-235 C).
At the end I recovered 93 g of reddish unreacted glycerol from the flask (if the heating was not so strong especially in the second serie I believe
there was much less of residual glycerol).
The exact amounts of reactants:
glycerol 330 g + 292 g = 622 g, so 529 g of glycerol reacted (5,75 mol)
85% formic acid 102,3 + 98,6 + 98,3 + 102 + 101 + 104,6 = 606,8 g which is 516 g of 100% formic acid which is 11,2 mol (seems half of the formic
acid reacted and half distilled out with the water from 85% acid and the water from esterification of glycerol with formic acid so it seems to be
worth of recovering formic acid from the distillate).
water + formic acid obtained as a distillate:
45 + 52 + 55 + 47 + 51 + 55 ml
Allyl alcohol obtained as a distillate:
105 + 105 + 72 + 92 + 82 + 65 ml
The 72 ml in the third round of the first serie was very likely caused by too strong heating (the heating for the 3rd round should be weaker than for
the 1st round as the amount in the flask is about half of the initial amount).
The second serie yield was significantly lower (92 + 82 + 65 ml) as I applied much stronger heating (in the first serie I was more cautious).
I did not determine the concentration of the allyl alcohol obtained. I did not perform work-up yet. I would like to break azeotrope by salting out
using K2CO3 which also neutralizes traces of formic acid and hydrolyzes allyl formate.
Attachment: 10.1002@14356007.a01425.pdf (249kB)
This file has been downloaded 2254 times
photos will follow soon
[Edited on 18-2-2020 by Fery]
|
|
|
Fery
International Hazard
    
Posts: 1108
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
reactants

apparatus - !!! note the hose from the vacuum take-off adapter must lead to the outside of the building !!!
 
first round: 330 g glycerol + 102,3 g formic acid

water distilling with formic acid
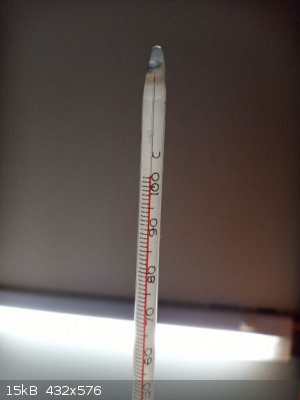  
about 45-55 ml of water obtained in one round

already collecting allyl alcohol as a distillate

allyl alcohol still passing as a distillate
  
allyl alcohol distillation going to finish for this round No. 1
  
round No. 2 by adding about 98,6 g of fresh 85 % formic acid into the glycerol left after the round No. 1 in the flask (temp of the glycerol about 100
C before addition of formic acid), then heating again

end of round No. 2 (note this thermometer is not so important, surprisingly small amount of allyl alcohol still passing through the condenser), the
important thermometer is the one submersed into the reaction mixture through the side arm of the RBF

end of round No. 2, here is the important thermometer, stop the heating when the temperature raises to 260 C

72 ml of crude allyl alcohol collected after the round No. 3 finished, best yields 105 ml (round No. 1, 2), worst yields like 65 ml in other rounds
when heating too strong (second serie) and literature claims also poor yield when heating too weak

remaining glycerol after the first serie (after the round No. 3) - will be used later in the second serie by adding new fresh pure colorless anhydrous
glycerol
 
second serie round No. 1 started (so the fourth round totally) by adding 292 g of fresh anhydrous glycerol to the remaing yellow glycerol from the
first serie + 102 g of 85 % formic acid and then heating again

the remaining 93 g of glycerol after second serie round No 3. (so totally 6 rounds) - no tar, just reddish color
 
[Edited on 18-2-2020 by Fery]
|
|
|
|