nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Energetic precipitate from the reaction of ascorbic acid/Cu(II) and sodium nitrite
First of all, I feel kind of stupid to return to SMDB, being 100% sure now that this board is monitored by law enforment. I quit making energetic
materials and do not own any chemical compounds nor information anymore that are illegal to possess. There is however no law against posting a 100%
legal reaction on this board, so I hope this will not spawn further intimidations.
For an experiment I needed some nitric oxide gas, so I made a nitric oxide generator from the reaction of a large excess of ascorbic acid with sodium
nitrite. When the nitric oxide production was measured however, yields were lower than theoretical, so I decided to try and add some copper sulfate as
a catalyst.
Experimental:
3 g of ascorbic acid was added to a 250 ml erlenmeyer together with 250 ml of distilled water and cooled to about 10 deg C. When the ascorbic has
dissolved, 0.5 g of copper sulfate pentahydrate was added, forming a light green solution. In very small amounts at the time, 0.5 g of sodium nitrite
was added over the course of about 30 minutes. The flask being lightly stoppered directly after the nitrite additions. During the additions some
nitric oxide (NO) was formed that escaped the solution (Do this outside or in a good fumehood!!!). During the additions the reaction mixture went from
light green to a distinct yellow colour. After nearly all of the sodium nitrite was added, quite suddenly large amounts of a chocolate-brown amorphous
precipitate started to form. This was allowed to settle for 30 minutes, decanted, filtered off into a pre-weighed filterpaper, washed with water and
finally 98% ethanol. The filter paper was allowed to dry fully and weighed, yielding 0.16-017 g of a dark brown compound. The filtrate was strongly
basified using sodium carbonate, which precipitated no additional copper, suggesting all was sequestered in the reaction.
The dry precipitate is very energetic and detonates in sub-mg amounts when heated on a spatula. It seems to gradually lose its energetic properties
when exposed to the air for several days, (more quickly so when wet) and transforms to a pitch black compound. A few mg of the dark brown precipitate
was added to a beaker and some dilute HCl was added. At first a white precipitate is formed, that dissolves again when more HCl is added, suggesting
the compound is some cuprous salt. A very acrid metallic smell was formed upon acidification, reminiscent of hydrazoic. Since all of the copper was
removed from the reation mixture, it seems to follow that the molecular weight of whatever is attached to the copper(I) is around 15-20 g/mol.
My best guess would be something like a double salt of cuprous oxide with cuprous fulminate (Cu(I)2O * CuCNO). If this is true this might be the
first reported direct synthesis of a copper fulminate. Not sure this is a fulminate though, any other ideas anyone about the product formed and
reaction mechanisms? 
This reaction seems very interesting, it seems amazing to me that oxidation and reduction reactions can seemingly coexist simultanously under these
conditions, maybe the strong copper(I) ligand interactions are helping here. So many new reactions spring to mind here, maybe also relevant to
diazenium diolate chemistry as well...
[edit] A further note if this does produce a fulminate: Fulminates and fulminic acid are INCREDIBLY toxic, about equal to cyanides. On
acidification of this precipitate or even during the reaction itself, fulminic acid or hydrocyanic acid could be released in quite large amounts. Do
not scale this up without adequate safety measures!!!!
[Edited on 13-11-2019 by nitro-genes]
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Very interesting compound! I found some material about Cu-nitrosyl, peroxynitrite and nitro complexes. Mayebe your compound is similar complex?
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2908284/
[Edited on 16-11-2019 by Bedlasky]
|
|
|
Metacelsus
International Hazard
    
Posts: 2543
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by nitro-genes  | Since all of the copper was removed from the reaction mixture, it seems to follow that the molecular weight of whatever is attached to the copper(I)
is around 15-20 g/mol.
My best guess would be something like a double salt of cuprous oxide with cuprous fulminate (Cu(I)2O * CuCNO). If this is true this might be the
first reported direct synthesis of a copper fulminate. Not sure this is a fulminate though, any other ideas anyone about the product formed and
reaction mechanisms? 
|
I'm not sure you can confidently say that all the copper was present in the precipitate. It's possible some remained in solution even after you added
the sodium carbonate (perhaps some ligand formed during the reaction kept it soluble). I think you should directly measure the amount of copper
present by decomposing the compound to some known copper complex.
Also, is the ascorbic acid necessary for the reaction? Or could another reducing agent substitute?
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
The affinity for copper(I) towards the formed NO as a ligand is probably helping here indeed. Even when quite a lot of nitrite is added at once, the
formed NO that escapes the solution is very quickly absorbed back into the reaction mixture. Since the energetic precipitate formed is quickly
destroyed under even mildly acidic condictions, I tried doing the reaction reverse, so slowly adding ascorbic acid to a Cu(II)-sodium nitrite
solution, though this does not produce any of the brown precipitate at all, suggesting that a high concentration of Cu(I) is needed for the reaction
itself. This would probably also mean that any other possible reducing agent instead of ascorbic would have to be capable of reducing copper(II) in
solution, yet not to copper metal and also not directly react with nitrite istelf, (like sulfites for example). Ascorbic may be pretty unique in this
regard. Since the precipitation of the compound may be pH determined (it precipitates at a pH around neutral), it might also be informative to find
the minimal amount of ascorbic/nitrite combination to establish how many moles of nitrite relative to the copper are needed.
After some reading... this thread may not even belong to organic chemistry at all!  Apparently, the presence of copper(I) in some instances may lead to dimerization and further reduction of the NO dimer produced, forming a
hyponitrite (also see. J. Am. Chem. Soc. 2017, 139, 38, 13276-13279 and Journal of the Chemical Society (1928): 1449-1455.) Ascorbic is a very strong
reducing agent, so this would definitely be possible. Supposedly, even boiling nitrate salts with ascorbic acid alone is able to produce nitrites
almost quantitatively. Apparently, the presence of copper(I) in some instances may lead to dimerization and further reduction of the NO dimer produced, forming a
hyponitrite (also see. J. Am. Chem. Soc. 2017, 139, 38, 13276-13279 and Journal of the Chemical Society (1928): 1449-1455.) Ascorbic is a very strong
reducing agent, so this would definitely be possible. Supposedly, even boiling nitrate salts with ascorbic acid alone is able to produce nitrites
almost quantitatively.
Hyponitrite istelf apparently is a very strong ligand towards copper (I) and forms a chocolate coloured and water-insoluble precipitate with a
copper(I) salt, even in the presence of a large excess of chloride ("Some reactions of hyponitrites and the related substances - part II, J.Indian
Chem.Soc" see atachment). On decomposition it may form nitrogen gas and copper(II)oxide --> Cu2N2O2 --> 2 CuO + N2 + a lot of energy (a
nanothermite of cuprous hyponitrite with aluminium or magnesium powder ought the be interesting!  ) . The latter reference also studied the thermal decomposition of cuprous hyponitrite and strangely though, they never
mention strongly energetic properties for their compound, so maybe the precipitate obtained from ascorbic/Cu/nitrite is something entirely different
after all...hmmm ) . The latter reference also studied the thermal decomposition of cuprous hyponitrite and strangely though, they never
mention strongly energetic properties for their compound, so maybe the precipitate obtained from ascorbic/Cu/nitrite is something entirely different
after all...hmmm 
As metacelsus says, analyzing the copper content of the brown precipitate would probably be more informative, though I don't see an easy way of doing
so, except heating it for a long time far under it's explosion temperature (around 210 deg C) and finally higher temperatures, which may leave
relatively pure copper(II)oxide...The precipitate also dissolves easily in dilute ammonia, so this may also be an option for some tests.

[Edited on 18-11-2019 by nitro-genes]
Attachment: Some reactions of hyponitrites and the related substances.pdf (1.8MB)
This file has been downloaded 572 times
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
If it is soluble in ammonia, try reducing by nascent hydrogen in alkaline media (as a source of hydrogen you could use Al or Zn and ammonia +
hydroxide solution). You end with metallic copper. I once tried reduce copper(II) hydroxide in excess of sodium hydroxide with aluminum foil and it
works.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Even though I can't see any direct use for these compounds, I was quite puzzled by the difference in energetic properties and stability of the
complexes obtained from different reaction conditions, so I did a few additional experiments.
It seems the largest influence of the reaction is the pH. Unfortunately, buffering the reaction using sodium acetate/acetic was not helpful, in most
cases no precipitate was formed at all.
At a pH of 5-6 using sodium ascorbate (or simply by using less ascorbic acid) a golden-brown complex is formed. This is very energetic and can
detonate in the smallest amounts when heated. Even at room temperature, it loses much of its energetic properties very quickly (several hours). The
remaining compound seems much more stable though and seems to contain hyponitrite. Due to the higher pH, the nitrite itself may act as a ligand and I
think the initially very energetic complex maybe contains some cuprous nitrite, which probably disproportionates very quickly after isolation into
hyponitrite and cuprous oxides, which are probably more stable. Information on the synthesis and composition of stable complexes of cuprous nitrite
seems very sparse, the only reference that was found referred to Gmelins handbuch of inorganic chemistry but the relevant pages could not be found
online.
When a large excess of ascorbic is added and the pH remains between 2-4 the entire reaction, a very dark reddish brown compound is formed in a
quantity (relative to the copper sequestered) which matches the molecular weight of cuprous hyponitrite almost completely. In this case, very little
nitrite is probably able to act as a ligand and nearly all the nitrite is converted to nitric oxide gas. Using an airlock (wine making), it was
evident the NO gas that escapes the solution initially is absorbed very quickly into back into the reaction mixture and probably dimerized and reduced
further to hyponitrous/cuprous hyponitrite. When well washed and dried, cuprous hyponitrite seems pretty stable and showed no discoloration or change
in reactive properties when stored for a week at room temperature in the open air. It starts to slowly decompose at around 130 C and puffs off
somewhat energetic at around 150-210 C.
When cuprous hyponitrite is dissolved in ammonia (or only catalytic amounts of ammonia are added to slightly wetted cuprous hyponitrite), the
copper(I) is oxidized to Cu(II) by air very fast. If the resulting deep blue solution is left to evaporate, a pea-green salt precipitates, probably
Cu(II)2(OH)2(N2O2). Similarly to cuprous hyponitrite, it starts to decompose at around 130 C, forming black cupric oxide.
Reducing the amount of ascorbic by substituting for an equimolar amount of acetic acid only yielded very small amounts of cuprous hyponitrite. Also,
when only a catalytic amount (a few mg) of copper(II) sulfate was added, the NO gas was only absorbed for the first 100 mg of nitrite or so and the NO
started escaping the solution after that, even though all copper remained in solution. Since the cuprous hyponitrite itself seems pretty stable, even
in 10% acetic acid, it seems most likely that the reaction depends on delicate ligand interactions mediated by Cu(I), that doesn't tolerate other
copper(I) ligands, including hyponitrous itself.
[Edited on 30-11-2019 by nitro-genes]
|
|
|
zed
International Hazard
    
Posts: 2285
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Gene most of us, are in the the US of A, and the harmless use of explosives, is fairly legal here.
Don't do things to hurt other people, or other peoples property, and you shouldn't have problems.
And, if LE doesn't monitor this sight occasionally, I would be surprised.
|
|
|
Boffis
International Hazard
    
Posts: 1897
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Given the conditions under which Brauer describes the preparation of hyponitrites (sodium amalgan reduction of sodium nitrate) I find it hard to
believe that it can be the reaction product of ascorbic acid and sodium nitrite. Many organic acids react with sodium nitrite to form weird compounds
that appear to be little related to the parent. Sorbic acid is one of the best studied because of its presence with nitrites in food. Products as
diverse as ethyl nitrolic acid and 1,4-dinitro-2-methylpyrrole can result. Soooo... I think hyponitrite looks like a fairly remote possibility,
however, the possibilities with ascorbic acid are considerable. For examples could the unsaturated nature of ascorbic acid allow the formation of a
transient pseudonitrosite? I can imagine that with hydroxy and nitroso groups on the same carbon atom they would be very unstable but what would the
decomposition products be? likewise the presence of hydroxy and nitro groups on the same carbon atom.
A few questions nitro-genes. Is this method of producing nitric oxide a known one with a published procedure? Why did you think that Cu would act as a
catalyst in the disengagement of NO? Have you tried other metals to see if they produce ppts (particularly silver and mercury)? Also why the very
dilute solutions?
[Edited on 2-12-2019 by Boffis]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
The chemistry of NOx is pretty complicated with all the different dimers and disproportionations possible...OH-NO-NO chemistry seems fitting 
Yes, the formation of nitric oxide from ascorbic and nitrite was published in some paper on diazenium diolate chemistry IIRC, I'll see if I can find
it again. Ag+ does produce a yellow precipitate with hyponitrites, though in the presence of ascorbic it is instantly reduced to metallic silver.
The mercury in the sodium amalgam preparation of the stable cis-form of sodium hyponitrite might be a necessary component of the reaction and not only
to moderate the reactivity of the sodium metal. An interesting question is also what the role of ascorbic is in the formation of the cuprous
hyponitrite indeed. The initial reaction of nitrous/nitrite with ascorbic acid has been described in several articles and is thought to occur via mono
and di O-nitroso intermediates (1: Wasserman et al, 1981), figure attachment). The authors from the latter paper also postulate direct formation of
hyponitrous via 2,3-dinitrosoascorbic ***, which could be competing with nitric oxide formation itself. Maybe a cuprous complex of intermediate III or
VIII is what is mainy responsible for the preferential formation of hyponitrite in the presence of copper? Though, could this also explain the
observed very quick absorption of NO gas and the lack of any cuprous hyponitrite formation with competing ligand present?  Would NO directly react with nano copper metal to produce cuprous hyponitrite? Would
leading NO gas into a copper sulfate/ascorbic mixture also produce cuprous hyponitrite? Maybe the ascorbic is just a sufficiently weak copper ligand
that it allows direct reduction of nitric oxide itself via a Cu(I) NO complex? Would NO directly react with nano copper metal to produce cuprous hyponitrite? Would
leading NO gas into a copper sulfate/ascorbic mixture also produce cuprous hyponitrite? Maybe the ascorbic is just a sufficiently weak copper ligand
that it allows direct reduction of nitric oxide itself via a Cu(I) NO complex?
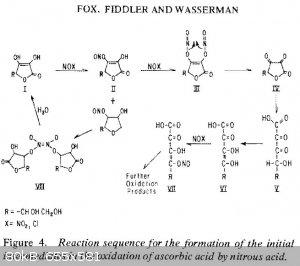
1. Fox Jr, Jay B., Rosemary N. Fiddler, and Aaron E. Wasserman. "Initial reaction intermediates in the oxidation of ascorbic acid by nitrous acid."
Journal of food protection 44.1 (1981): 28-32.
*** Formation of an internal vicinal nitroso dimer from nitrosoacorbic acid would account for the absence of an ascorbyl radical during the reaction.
We propose that the electron transfer reaction begins with the further nitrosation of the nitrosoascorbic acid intermediate to form the 2.3
dinitrosoascorbic acid, II ....... III. A two electron transfer then takes place to yield dehydroascorbic acid, IV, and N202. The electron transfer
would be facilitated by formation of the dimer, a stable form of NO (26)."
[Edited on 2-12-2019 by nitro-genes]
|
|
|
Boffis
International Hazard
    
Posts: 1897
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi nitro-genes; had a look at the original paper. Mmmm they talk about nitrosoascorbic acid but in reality they are talking about alkyl nitrite
esters. This may be irrelevant but I can't help feeling that their data doesn't preclude the formation of pseudonitrosite and their compound VIII is
very much like the dimers produced by pseudonitrosite.
Any how I still don't see how hyponitrite is product unless by the addition of electron to the N2O2 molecule they postulate being formed by the break
up of the dimer. Is ascorbic acid capable of such a reduction? There is some interesting reading in the references that I will have to have a look at.
Addition:
While rumaging through some old chemistry books I came upon references to hydroxamic oximes. These contain the funtional group -(HONH)-C=NOH and form
characteristic reddish or brown copper salts (no claims about valency of Cu). Given that nitrite can cleave the double bond in sorbic acid to give
ethyl nitrolic acid I wonder if similar reactions are not possible with ascorbic acid.
[Edited on 3-12-2019 by Boffis]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Any references for the hydroxamic oximes? Seems like interesting reading material. 
The O-nitroso ascorbic derivatives are probably like very unstable alkylnitrites indeed, the attached NO+ being very quickly reduced to NO (g),
leaving dehydroascorbic. In contrary to my first post... with the large excess of ascorbic present and absence of oxygen (air lock) I don't see large
amounts of these pseudonitrosites being produced anyway (also see: Williams, Daniel Lyn Howell. Nitrosation reactions and the chemistry of nitric
oxide. Elsevier, 2004, .p112-p114) Going from ascorbic or the hydrolysis products it seems only oxidation to carboxylic acid and finally CO2 seems
likely. The best argument against this would also be the observation that the pea-green salt formed after air oxidation of the presumed cuprous
hyponitrite itself is not energetic at all and also matches descriptions of the Cu(II)(OH2)(N2O2) in literature.
Would be interesting to test whether leading NO (g) into some nano-copper metal + maybe a little NaCl would produce cuprous hyponitrite. Or maybe
simply leading NO (g) into an aqueous solution of Cu(II) sulfate + 0.5 mole eqvt ascorbic would produce cuprous hyponitrite. If so, using these
amounts of ascorbic acid, such a synthesis might be competitive with other synthesis routes for sodium hyponitrite, since IIRC having read that
cuprous hyponitrite + 2 mole eqvt NaOH produces a precipitate of cuprous hydroxide and a solution of reasonably pure sodium hyponitrite. Even the
synthesis routes going from the sodium mercury amalgam give like 18% yield IIRC. While most direct routes only produce the unstable cis-forms of
sodium hyponitrite.
Seen the volume of gas produced upon introducing the nitrite during the reaction, I'd really guess the production of the presumed hyponitrite is
originating from the NO (g) btw and not from some O-(di)nitrosoascorbic. This would need some further testing though...
[Edited on 5-12-2019 by nitro-genes]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Another random thought... From the article on cuprous hyponitrite attached above, it seems as though the authors are hinting towards the difference
and interchangeability between the cis and transforms by their observations on the thermal decomposition of cuprous hyponitrite. Would also be
interesting therefore maybe to see whether the unstable cis isomer of sodium hyponitrite would be able to spontaneously revert to the more stable
trans-form by forming a transition metal complex/salt.
[Edited on 5-12-2019 by nitro-genes]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Some thoughts, on possible chemistry which may help.
First, from "Fenton chemistry in biology and medicine" by Josef Prousek, to quote reaction (15) on page 2330, a general depiction of Fenton-type
reactions, to quote:
"For Fe(II) and Cu(I), this situation can be generally depicted as follows [20,39],
Fe2+/Cu+ + HOX → Fe3+/Cu2+ + ·OH + X- (15)
where X = Cl, ONO, and SCN. "
Assuming HOX could also represent HONO, possible reactions in the current context:
Cu+ + HONO → Cu2+ + ·OH + NO-
Or:
Cu2+ + HONO → Cu3+ + ·OH + NO-
where NO- is more correctly depicted as hyponitrite anion, N2O2(2−). The presence of hydroxyl radical suggests ·NO formation via:
NO- + ·OH = ·NO + OH-
as was reported.
Also, ascorbic acid is known to provide some recycling of ferric and cupric ions back to ferrous and cuprous to keep a fenton-type reaction active (as
a reference, see, for example, "Generation of Hydroxyl Radicals from Dissolved Transition Metals in Surrogate Lung Fluid Solutions" by Edgar Vidrio,
et al at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2626252/ ).
Also, some support per the comment by Nitro-Genes:
Quote: Originally posted by nitro-genes  |
.........
After some reading... this thread may not even belong to organic chemistry at all!  Apparently, the presence of copper(I) in some instances may lead to dimerization and further reduction of the NO dimer produced, forming a
hyponitrite (also see. J. Am. Chem. Soc. 2017, 139, 38, 13276-13279 and Journal of the Chemical Society (1928): 1449-1455.) Apparently, the presence of copper(I) in some instances may lead to dimerization and further reduction of the NO dimer produced, forming a
hyponitrite (also see. J. Am. Chem. Soc. 2017, 139, 38, 13276-13279 and Journal of the Chemical Society (1928): 1449-1455.) |
Also, a possible reaction of the hydroxyl radical with any present organic, for example, ethyl alcohol, with the creation of the ethyl radical, which
could further, with say ·NO, combine.
My research suggests some created organics hyponitrite are explosive, like ethyl hyponitrite (see https://books.google.com/books?id=8PYZAAAAIAAJ&pg=PA317&... ) and also to quote Wikipedia at https://en.wikipedia.org/wiki/Hyponitrous_acid on hyponitrous acid :
"Trans-hyponitrous acid forms white crystals that are explosive when dry" .
which all suggests some explosive ideas.
[Edited on 7-12-2019 by AJKOER]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
The described salts and organic esters of hyponitrites are of no use as explosives, due do their instability. The only patented use of organic
hyponitrites is the hyponitrite ester of tertbutanol, as a radical initiator in polymerization reactions. These are fairly old patents from the
1980's... no idea if hyponitite esters are still used nowadays.
Leading an excess of NO (g) into a copper sulfate + 1 mol eqvt ascorbic did not produce any precipitate of cuprous hyponitrite. Unfortunately I
couldn't see if the NO was absorbed or not. When the reaction mixture was basified dropwise using NaOH afterwards, only cuprous hydroxide/oxide
precipitated. I guess the hyponitrite formed from the reaction of ascorbic /Cu(II) and nitrite occurs through a cuprous complex of one of the
O-nitrosoascorbic derivatives afterall. Would be interesting if a cheaper ketone or something would be suitable or be able to act as a catalyst.
[Edited on 8-12-2019 by nitro-genes]
|
|
|
Boffis
International Hazard
    
Posts: 1897
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I've done a bit more research and I now see that the stoichiometry is 1 mol. ascorbic acid and 2 mols. sodium nitrite/nitrous acid gives 1 mol. of
dehydroascorbic acid and two mols. of nitric oxide. But in nitrogenes initial experiment the ratio of reactants wasn't 1:2 it was 2.37:1 therefore
there was a vast excess of ascorbic acid acid. They may explain why you see both oxidation (ascorbic acid to dehydroascorbic acid) and reduction
reactions (Cu2+ to Cu+) at apparently the same time. So the question now is: can the excess ascorbic acid reduce nitric oxide to hyponitrite?
This of course assumes that the nitrous acid and ascorbic acids still react with the same stoichiometry when the latter is present in large excess.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
An alternate view would be there is only reductions going on, another likely reason this is some cuprous hyponitrite or complex thereof, since it is
the simplest explanation. I've made a lot of suggestions about the mechanisms (out of interest) in this thread, though it is really surprising to see
how seemingly simple reactions can be quite complicated mechanistically, or at least to really prove anything in this regard using experiments.
The more than 2 molar excess of ascorbic could maybe point towards a cuprous complex of compound VIII as the most likely precursor, or maybe the lower
pH is a requisite for formation of the nitroso ascorbic intermediates in general or, or maybe there just needs to be a large excess of reductant
present for other reasons. Then there is the observed absorption of NO (g) which still puzzles me somewhat...more questions than answers as
usual...
[Edited on 10-12-2019 by nitro-genes]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Maybe using an alkylnitrite could work more efficient to produce cuprous hyponitrite? Supposedly, ethyleneglycol dinitrite is somewhat water soluble,
though probably a sensitive explosive, so very dangerous to handle. Does ethylene glycol mononitrite also exist? A mononitrite of any poly-ol would be
far more water soluble I guess? Maybe reduction of aqueous or partly alcoholic solutions/emulsions of an alkylnitrite (or a more watersoluble
mono-nitrite of a poly-ol) using copper(II)sulfate and a slight theoretical excess of ascorbic acid could work to produce cuprous hyponitrite more
efficient? Even at slightly acidic pH the alkylnitrite would slowly hydrolyse producing a steady and pH neutral supply of HNO2.
It seems that already at a pH of about 5, cuprous hydroxide/oxide is already formed to some extend. The low pH seems like a necessity for pure cuprous
hyponitrite also simply to prevent cuprous hydroxide/oxide co-precipitation). The excess ascorbic might also be needed just to keep the pH low enough.
Pitty that adding small equimolar amounts of other acids (acetic/sulfuric/HCl) seems to have a very detrimental effect on the reaction.
The organic nitrite therefor seems like an interesting test at least, the alcohol would be (I guess) neutral as a ligand itself and may provide some
clue whether the ascorbic excess is actually needed as a reaction precursor itself, or in maintaining pH/Cu(I) pressure/stability, or preventing
oxidation of the hyponitrous formed.
[Edited on 12-12-2019 by nitro-genes]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Tested on a small scale if the presumed cuprous hyponitrite would react with excess NaOH to produce cuprous oxide and leave a very basic solution of
sodium hyponitrite. About 0.2 g of cuprous hyponitrite was added to a 25 ml erlenmeyer together with 25 ml dH2O and 0.3 g NaOH was added. The flask
was stoppered airtight and stirred for 3 hours at 15 C. The colour of the precipitate gradually changed to a bright orange. This was allowed to settle
overnight at 4 C, and the clear supernatant pipetted off into a 50 ml erlenmeyer.
Silver hyponitrite was then prepared according to: "Partington, James R., and Chandulal C. Shah. "384. Hyponitrites. Part II: metallic salts. Part
III: esters." Journal of the Chemical Society (Resumed) (1932): 2589-2597".
With strong stirring 0.25 g silver nitrate in 4 ml dH2O was added dropwise to the supernatant. A transient brownish coloured precipitate forms, that
changes very quickly back to a yellow. The bright orange precipitate is not energetic and seems likely entirely composed of curpous oxide.
Seems that the hyponitrite content of the cuprous hyponitrite can perhaps be titrated using the silver salt (planning this on larger scale), since I
wonder if the initial complex formed from ascorbic/Cu(II)SO4/sodium nitrite might not be something like Cu2N2O2 * H2N2O2.
In some patents on the synthesis of the organic esters of hyponitrites (or diazenium oxides due to ambident nucleophilicity) the use of silver
hyponitrite in combination with alkylhalides seems preferable. Would cuprous hyponitrite similarly be able to react with alkyl halides directly? Alkyl
halide + cuprous hyponitrite --> alkyl hyponitrite + cuprous halide. The latter would be insoluble and easily filtered off if this would work,
though the copper might also form more complex products as opposed to silver salts? Any ideas? 
[Edited on 20-12-2019 by nitro-genes]
|
|
|
|