| Pages:
1
2 |
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Vacuum Oven vs Kiln for Molecular Sieve Drying
I use plenty of 3A and 4A molecular sieves, mostly to dry alcohols.
Unfortunately, the reactivation is an ordeal as I can use only a 2L heating mantle for that - I usually VERY slowly heat it up to prevent melting of
the glass and even then it takes few hours until last droplets of water escape. Then I pump out the air which releases more water. The whole procedure
is very time consuming. I though about running dry nitrogen or argon slowly through the flask to drive the vapour off to speed things up a bit.
I wonder if one can use kiln instead as this provides high enough temperatures for drying sieves at atmospheric pressure (300 °C and more) and many
kilns can be programmed for convenience.
Vacuum ovens reach lower temperatures (only about 250 °C), but enough for drying sieves under vacuum (I assume temperatures over 200 °C and pressure
of 100 mbar is sufficient). Vacuum ovens are maybe more useful as I can use them to dry sensitive solids, glassware etc.
I am also thinking about some makeshit solution like putting sealed evacuated flask with sieves in ordinary oven set at max temperature (220-250 °C),
leave it there and periodically flush it with dry gas to remove the released water vapour.
What would be your choice for drying molecular sieves?
I don't need them absolutely dry since I don't do any moisture-sensitive work, usually just need to break ethanol-water azeotrope or pre-dry solvents
for precipitation of hygroscopic salts.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
You can simply use a microwave 
Nuke them 2min and you're good.
[Edited on 10-7-2019 by karlos³]
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by nimgoldman  | I use plenty of 3A and 4A molecular sieves, mostly to dry alcohols.
Unfortunately, the reactivation is an ordeal as I can use only a 2L heating mantle for that - I usually VERY slowly heat it up to prevent melting of
the glass and even then it takes few hours until last droplets of water escape. Then I pump out the air which releases more water. The whole procedure
is very time consuming. I though about running dry nitrogen or argon slowly through the flask to drive the vapour off to speed things up a bit.
I wonder if one can use kiln instead as this provides high enough temperatures for drying sieves at atmospheric pressure (300 °C and more) and many
kilns can be programmed for convenience.
Vacuum ovens reach lower temperatures (only about 250 °C), but enough for drying sieves under vacuum (I assume temperatures over 200 °C and pressure
of 100 mbar is sufficient). Vacuum ovens are maybe more useful as I can use them to dry sensitive solids, glassware etc.
I am also thinking about some makeshit solution like putting sealed evacuated flask with sieves in ordinary oven set at max temperature (220-250 °C),
leave it there and periodically flush it with dry gas to remove the released water vapour.
What would be your choice for drying molecular sieves?
I don't need them absolutely dry since I don't do any moisture-sensitive work, usually just need to break ethanol-water azeotrope or pre-dry solvents
for precipitation of hygroscopic salts. |
Putting a sealed flask containing a significant amount anything damp/wet in a 300c oven is probably a bad idea. Thing about the vapour pressure of
water at 300C.
Usually the limitation of drying a powder or granules is the low thermal conductivity of them in combination with the need to supply heat to vaporize
the liquid. So it inevitably take time.
Spread the granules out on a large tray so the depth is as shallow as possible put them in a clean oven at the recommended temperature and forget
about them for a hour or more or ideally until they are a constant weight.
I would expect if you can pull a constant vacuum while heating the granules it would reduce the time significantly.
PS Microwaving the granules can be problematic. While they are damp the vaporization of the liquid will keep them no hotter than about 100C but once
they are dry the microwave energy is still going into the oven and could even melt them. So the time required would be dependent the amount liquid to
be evaporated.
I have on occasion dried item of cotton clothing in a microwave but the item has to be monitored almost contumaciously to avoid over heating as they
approach dryness or the item will be scorched or even ignited.
I have also accidentally melted manganese oxides/carbon from a used dry cell while drying it in a microwave oven. Doing that deliberately is
interesting.
[Edited on 10-7-2019 by wg48temp9]
[Edited on 10-7-2019 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by nimgoldman  |
I am also thinking about some makeshit solution like putting sealed evacuated flask with sieves in ordinary oven set at max temperature (220-250 °C),
leave it there and periodically flush it with dry gas to remove the released water vapour. |
this seems even more work.
you said that you heat the seaves and THEN youu vacuum it, why not doing it at same time? (lack of convection so uneven heating?) heat the flask while
connected to a vacuum pump with a hose adapter (running continuously or on a cycle with a timer). if you need more volume for larger batches you could
convert a stainless steel pot to a vacuum chamber (similar to those sold as vacuum chambers for degassing silicone) by adding a steel disk on top with
the right hose fitting and a gasket that survuves 250 or 300 degrees. or maybe a pressure cooker with a hole tapped on the lid for a hose adapter, it
already has a heat resistant gasket (maybe not for 300°C though)
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
I see.
So perhaps I will pre-dry the sieves on tray in a simple electric oven (not microwave) for some hours (maybe 2-3 hrs?) then place them in RBF in a
mantle for final drying under vacuum.
Once problem I had with vacuum drying is that my diaphragm pump pumps lots of hot water vapour which of course condenses. The pump has a reservoir but
maybe it's better to remove bulk of the water first.
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
i use old pressure cooker with induction heating connected to vacuum pump.bunch of steel balls to even out heating .
also before any of that simple distill the residual alcohol.
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by nimgoldman  | I see.
So perhaps I will pre-dry the sieves on tray in a simple electric oven (not microwave) for some hours (maybe 2-3 hrs?) then place them in RBF in a
mantle for final drying under vacuum.
Once problem I had with vacuum drying is that my diaphragm pump pumps lots of hot water vapour which of course condenses. The pump has a reservoir but
maybe it's better to remove bulk of the water first. |
Vacuum drying at 250C after heating in an open tray at 250C will not dry the sieves significantly more so its not required particularly as your only
using them to break an azeotrope.
At 250C the vapor pressure of water is about 40bar at 300C its about 86bar.
So adding even a perfect vacuum will only add an additional 1bar.
Putting it a different way you could get the same effect as the vacuum by increasing the temperature by a few degrees.
I checked about 250+3C or 300+1C for an extra one bar.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
monolithic
Hazard to Others
  
Posts: 436
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Honestly, just drying them in a normal oven at 250 C for 3 hours is good enough. Will they be bone dry and 100% activated? Probably not. Will they
work well enough for the vast majority of cases? Yes.
|
|
|
Pumukli
National Hazard
   
Posts: 705
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Isn't it dangerous to put a few litres of wet sieves into a regular oven at high heat? I mean explosion hazard.
The op used them to dry alcohol(s), so they not only contain water but some alcohol as well.
Experience?
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Since you mentioned using nitrogen gas, if you have a house supply of dry nitrogen (some labs have it piped in) you can try drying the sieves by
flushing them for some time in a column at room temperature. I don't know how well this works for molecular sieves, but it does work for silica gel.
The indicating gel turns blue again after being flushed with dry nitrogen, even at room temperature.
I did buy some 3A sieves, but I took them home earlier, so I don't have them here with me in the lab. Otherwise I'd try this out.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Unfortunately the sieves won't release water so easily as the H2O molecules are "stuck" in the zeolite crystals. It takes temperatures of 250 °C or
so until they release the water.
As for the alcohol, I usually pre-dry the sieves with low heat (e.g. food dehydrator) and then apply high heat. When I use oven, I have the oven fan
turned on so any residual alcohol vapours are flushed.
I am drying about 750 g of sieves at once, but will probably do more in future.
|
|
|
monolithic
Hazard to Others
  
Posts: 436
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Pumukli  | Isn't it dangerous to put a few litres of wet sieves into a regular oven at high heat? I mean explosion hazard.
The op used them to dry alcohol(s), so they not only contain water but some alcohol as well.
Experience? |
Wash them several times with water and let them air dry for 24 hours -- they should no longer smell of solvent. I've done this with sieves soaked in
DMF and methanol for weeks at a time. They didn't blow up my oven.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Quote: Originally posted by rockyit98  | i use old pressure cooker with induction heating connected to vacuum pump.bunch of steel balls to even out heating .
also before any of that simple distill the residual alcohol. |
Omg why have I never thought to use my large heavy never otherwise used pressure cooker as a vacuum oven!!
Thanks!
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
The steel balls are a pretty good idea!
I've come out with my "standard procedure" like this so far:
remove any adhering solvent from sieves by either washing them with distilled water or by distillation
pre-dry the sieves using regular oven set at max. temp.; pre-dry the RBF
place sieves in RBF, flush with nitrogen/argon
heat up RBF in a heating mantle up to 300 °C (careful about overheating) or until you see enough condensate in the flask
pull vacuum, leave under vacuum for a few minutes
put under nitrogen/argon by e.g. attaching a baloon and releasing stopcock
repeat 4-6 several times
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Panache  | Quote: Originally posted by rockyit98  | i use old pressure cooker with induction heating connected to vacuum pump.bunch of steel balls to even out heating .
also before any of that simple distill the residual alcohol. |
Omg why have I never thought to use my large heavy never otherwise used pressure cooker as a vacuum oven!!
Thanks! |
Most pressure cookers have a gasket that has a V profile with the top of the V facing in to the pressure cooker. When the internal pressure pressure
is greater than the external pressure the sides of the V are forced apart to form more of U profile and seal against the lid and base.
With an internal vacuum the higher external pressure will tend to collapse the V and cause it to leak or possibly force part of the gasket into the
pressure cooker.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by nimgoldman  | The steel balls are a pretty good idea!
I've come out with my "standard procedure" like this so far:
remove any adhering solvent from sieves by either washing them with distilled water or by distillation
pre-dry the sieves using regular oven set at max. temp.; pre-dry the RBF
place sieves in RBF, flush with nitrogen/argon
heat up RBF in a heating mantle up to 300 °C (careful about overheating) or until you see enough condensate in the flask
pull vacuum, leave under vacuum for a few minutes
put under nitrogen/argon by e.g. attaching a baloon and releasing stopcock
repeat 4-6 several times
|
If your going to fill a regular laboratory flask containing possibly damp sieves with a gas at near room temperature, seal it and then heat it to
300C then when the gas expands and the damp sieves release water vapor the stopper will probably pop or worse.
Hopefully your stopper is sufficiently leaky or loose to prevent too large a pressure build up.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Pumukli  | Isn't it dangerous to put a few litres of wet sieves into a regular oven at high heat? I mean explosion hazard.
The op used them to dry alcohol(s), so they not only contain water but some alcohol as well.
Experience? |
I was regenerating 3A mole sieves used to dry ethanol. I placed the apparently dry sieves in a large glass cake pan in my kitchen oven at 400F. I
then went into the next room to watch TV. After a while I heard a loud bang! I concluded that the ethanol vapor had exploded and pushed open the
spring loaded oven door, then it self-closed. I continued the drying.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Below is snip of a set of isotherms for the absorption of water by 3A sieves.
You have to multiply the vertical axis by 1.8 to convert it to percent by mass. Unfortunately the pressure scale only extends to 0.3 of atmospheric
pressure. A room temperature isotherm is not shown. That would be be similar to the 100C isotherm but with a sharper knee approaching 11.7 mol/kg
(21% H2O by mass).
Interestingly at a pressure of 0.3 bar drying wet sieves at a temperature 200C only reduces the water content to about 9% from 21%. Note that the
attached paper suggested regeneration temperature above 270C irreversibly reduces the absorption ability of the sieves.
At 200C to reduce the water content to less than 1% it requires pressures less than 10mbar. Flushing with an inert dry gas that has a negligible
partial pressure of water vapor would be equivalent to reducing the pressure to zero which would remove that last 1% of moisture eventually.
Just drying drying the sieves at 200C give you about half the maximum drying capacity which is about 10% by mass. So to compensate for not using
totally dry seives the amount used just need to be doubled.
.
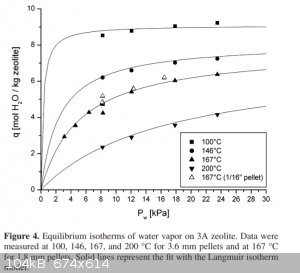
From http://fennetic.net/irc/zeolite_adsorption_curves.pdf
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Isn't that "Pw" the partial pressure of water in the drying air?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Yes if the sieves are in air and in equilibrium with it. ie the water vapor pressure of the sieves.
How can I relate those isotherms to drying alcohol? I guess a mixture of alcohol and water has a water vapor pressure. Assuming the alcohol does not
effect the capacity of sieves then at equilibrium the water vapor pressure of the sieves and the alcohol would be equal. I could approximate
the water vapor pressure of the mixture using Raoults Law.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
From my psychrometric chart: at 68°F (20°C) and 50% relative humidity the vapor pressure of water in air is 0.18 psia = 1.2 kPa.
If the air is heated to 200°C the water content will stay the same and therefore its partial pressure will remain at 1.2 kPa. But the relative
humidity will then be very low.
Therefore, from the isotherm at 200°C the water content of the zeolite will be 0.5mole H2O/kg, or 0.9%.
Does this look right?
[Edited on 1-9-2019 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Magpie  | From my psychrometric chart: at 68°F (20°C) and 50% relative humidity the vapor pressure of water in air is 0.18 psia = 1.2 kPa.
If the air is heated to 200°C the water content will stay the same and therefore its partial pressure will remain at 1.2 kPa. But the relative
humidity will then be very low.
Therefore, from the isotherm at 200°C the water content of the zeolite will be 0.5mole H2O/kg, or 0.9%.
Does this look right? |
No, its incorrect.
Your first paragraph may be correct.
Your second paragraph is incorrect. The result depends on how the air is heated. If it is heated at constant volume then the percent of water in the
air will remain the same as will it its amount per unit volume. The pressure will increase and the humidity may change. If you heat the air and allow
it to expand the ratio of water to air will remain the same and the amount of water per unit of volume will decrease. The humidity may change.
The third paragraph's "therefore" is in correct, that statement does not follow from paragraph 2.
The isotherms assume the sieves are in equilibrium with the water vapor surrounding them. That means at a given temperature the vapor pressure of
water from the sieves is the vapor pressure of the water in the air surrounding the sieves and that will determine the percentage of water in that
air, again at equilibrium.
What was the purpose of your question? Testing your understanding or mine?
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The purpose of my question is to try to determine what really happens when heating the zeolite in ambient air.
The ambient air at 20C will expand during the heating to 200C and much will leak out to maintain ambient pressure. This will decrease the amount of
water in the oven air. Therefore the partial pressure of the water in the oven air will be even lower than I stated above. This will allow the
zeolite to dry even further than the 0.9% water.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
If you heat saturated sieves in a oven say in a long necked flask to 200C.
As the the temperature of the sieves increases their water vapor pressure will at some point exceed that of the atmosphere and steam will escape from
the flask. Eventually the water content in the sieves will reduce until their water vapor pressure is equal to atmospheric pressure and the flask will
be filled with water vapor. Unfortunately the isotherm I showed does not go up to 100kPa but it can be estimated from metaly extrapolating the graph
to 100kPa. I estimated the percent water in sieves at that point as 11%
If the sieves are heated on tray in open air so that the reach equilibrium with the water vapor pressure of the atmosphere. then taking your figure of
1.2kPa at that pressure I estimate the percent of water in the sieves to be about 1.3%.
In practice the content of water will be between 1.3% and 11% depending on how well the sieves reach equilibrium with the atmospheric water vapor. If
heated in an oven the air needs to be changed with fresh air to prevent a build up of water vapor. Most domestic ovens do not do that and the result
will be closer to 11%.
One thing that surprised me was how effective flushing with a dry inert gas would be.
Combination MO when microwaving flush some cooling air through the oven but when used as a heated oven only they usual activate a flap that stops most
of the cooling air flow thru the oven. Such an oven can be modified to allow some flow. As has already been stated using microwaves to heat the
sieves risks over heating them.
The simplest and effective method would be to flush the sieves with heated air at 200C.
[Edited on 9/1/2019 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
So an air fryer would be more effective than a conventional oven?
Put the sieves in a shallow dish and let the hot air swirl around them.
https://www.thekitchn.com/how-do-air-fryers-work-265185
|
|
|
| Pages:
1
2 |