ChemNub
Harmless

Posts: 6
Registered: 9-6-2010
Member Is Offline
Mood: No Mood
|
|
Electrolyte Conductivity
Hi guys!
Recently I've been playing with some electrolyte solutions, trying to determine adequate conditions for making some metal salts, plating, reducing and
the like..
Unfortunately however, I can't get good conductivity in my solutions- even with fairly concentrated strong acids!
5% H2SO4 was reading at about 4.5k ohms (! yes 4000!), and 20% H2SO4 at 5k (makes no sense with my limited knowledge of conductivity..).
Magnetic stirring seemed to drop the resistance temporarily but it just crawled back up over the course of a few minutes. All the connections were
good, and the multimeter read the resistance of itself (~0) and a 50Ohm resistor (50.5) with pretty good accuracy..
I am seriously wondering if it's a problem with the multimeter however, as it does not report amperage correctly under any circumstances (perhaps a
fuse was blown at some point?) - reading 0.0mA with 12v going through 50Ohms.. (voltage readings tested with a computer PSU seem quite accurate)
Something is definitely amiss... Have I finally lost my mind? It's not necessary to become a little Tesla, sucking kilovolts out of the grid, just to
harness a few amps to dissolve a nickel.... is it?
|
|
|
ChemNub
Harmless

Posts: 6
Registered: 9-6-2010
Member Is Offline
Mood: No Mood
|
|
well, I tried a 15% HCl solution and was able to measure about 20 ohms =D
but still.. why would sulfuric acid be soo much less conductive? is this typical?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Electrode potentials perhaps? Electrochemistry is non-ohmic. In principle, no current flows until the redox potential is reached. Beyond the
threshold, current increases exponentially, the same as it does for current flow across a semiconductor junction. Ionic mobility is orders of
magnitude less, so there's only a narrow range where it looks exponential, since the resistance swamps it even at low currents.
In short, can you measure with a higher voltage, like the current drawn from 3 or 5V?
Tim
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@12AX7 "Electrochemistry is non-ohmic." Is this true? I don't see it this way. Conductivity units are reciprical resistance units (mhos). Sulfuric
solutions are slightly buffered and have an order of magnetude less ionization than HCl solutions. I won't work out how this would affect a
multimeter reading. Try a conductivity probe and see what that reveals.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Is this true? I don't see it this way. |
The point is that V=IR doesn't hold true, so the apparent resistance will be different depending on what voltage you are applying (and it therefore
doesn't make much sense to think of the electrolyte as having some fixed value of resistance anyway). Ohm's law doesn't apply, thus non-ohmic...
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Yes, it's true. Go read up
on the topic of electrode polarization, for example. There's some non-trivial behavior at the electrode-electrolyte boundary. There's charge
buffering, so in part it acts similar to a capacitor. There's also threshold conduction, so in part it acts analogously to a forward-biased diode. In
particular, the equivalent circuit of an electrode pair in electrolyte is not just a resistor.
|
|
|
bquirky
Hazard to Others
  
Posts: 316
Registered: 22-10-2008
Location: Perth Western Australia
Member Is Offline
Mood: No Mood
|
|
dont forget that electrolyte temprature will efect conductivity as those crazy little ions (you just never know what there going to do next  can move around more when its hot.. can move around more when its hot..
And differences of electrode size, metal type, cleanlyness/oxidisation state and evan the depth of the electrode if there is a concentration or
temprature gradiant present can cause stray potentials that can mess up resistance readings of a typical DMM
ive got a CuSO4 plating bath running at the moment conducting 10amps and droping about 4 volts which whould make the whole setup cables,connectors,
electrodes,electrolyte etc about half a ohmm
but that specific number is not relly relevent becuse it depends mainly on the area of and distance between the electrodes.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
bquirky summarised it nicely - electrode spacing, material + electrolyte composition, electrolyte temperature, applied voltage; all affect measured
conductivity. And if you're not using AC to measure with, electrolyte reactions can also be important, often resulting in changing measurements.
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
An electrolysis cell is highly nonlinear and Ohm's law certainly does not apply to it. A much better electrical equivalence model for an electrolysis
cell is a non-linear resistor with the Butler-Volmer characteristic.
I did an experiment with an electrolysis cell and measured the current as function of voltage. Read the following web page I made about this
experiment:
http://woelen.homescience.net/science/chem/exps/electrolysis...
At low voltages the cell may take a low current and then its resistance appears to be very high.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The resistance measuring in multimeters is designed for resistors that obey the Ohm law. The testing potential is often no more than 1V or thereabout.
Some multimeters even run on a single 1.5 V battery, so you can be quite sure their testing potential is not higher than that. On the other side, the
sum of overpotentials of two electrodes immersed in aqueous H2SO4 is commonly higher than 1V (unless one of the electrodes is dissolving and you get a
galvanic cell instead of an electrolytic one).
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Yes. The other point of my statement was, you'll get a fairly reasonable measurement (if not physically significant, at least useful), at higher
voltage (3-5V), which is sufficiently in excess of whatever electrode polarization and reaction potentials (< 1V) to reveal the bulk resistivity of
the cell.
Same thing happens to a silicon junction diode, where you get essentially perfect exponential behavior below 0.7V or so (from pA to mA -- nine orders
of magnitude!), turning to resistive at higher levels (due to the resistivity of the bulk silicon layers in the device). A 1N4001 might drop 1V at
1A, 1.5V at 14A and 2V at 30A (peak), which is almost linear, whereas if these points were exponentially related, the voltages would be much closer.
Tim
|
|
|
merrlin
Hazard to Others
  
Posts: 110
Registered: 3-4-2009
Member Is Offline
Mood: No Mood
|
|
Electrolyte conductivity measurements can be be made using an AC signal in place of a DC signal. This helps avoid the problem of redox potentials and
charging of the double-layer capacitance. I suggest using a variable transformer and/or a resistor divider with your household voltage to obtain a few
tenths of a volt at 50 or 60 Hz. You can then use your multimeter(s) to obtain the I-V characteristic of the cell. Large electrodes also help avoid
the capacitance charging problem. Highly conductive electrolytes will allow the capacitance to charge quickly, so small voltages and large area
electrodes will be required. For accurate measurements you want the capacitance large enough so that most of the potential is dropped across the
electrolyte and not across the capacitance. If you have a higher frequency AC source available, it can also provide greater accuracy, but may
complicate the I-V measurements.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
That is right.
Not only can but should be made in this way. In general, all conducivity values from tables are given for f=1000 Hz (standard) or are
extrapolated for f going to infinity.
Double-layer capacity is of order 1 microF/cm2, so measurements for 50 Hz can be not very accurate, especially for concentrated electrolytes.
For measurements of small AC voltages, simple but very useful electronic rectifier based on OP can be made. Example:
http://en.wikipedia.org/wiki/Precision_rectifier
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Thanks for schooling me up on this!
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ChemNub  |
5% H2SO4 was reading at about 4.5k ohms (! yes 4000!), and 20% H2SO4 at 5k (makes no sense with my limited knowledge of conductivity..).
|
Maybe it makes no sense but can be true 
See a picture from book "Fundamentals of electrochemistry"(2006) by Bagotsky.
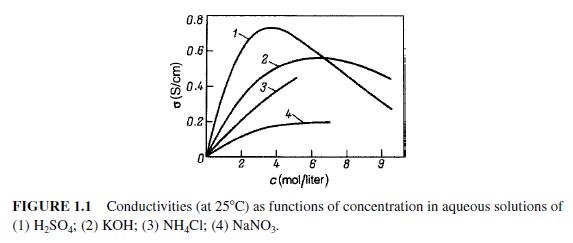
|
|
|