phakt
Harmless

Posts: 1
Registered: 24-3-2019
Member Is Offline
|
|
phenyl ring and piracetam
Hello,
given someone need phenylpiracetam and only have piracetam, is it possible to add a phenyl ring into piracetam?
how can that be done? any simple way?
[Edited on 25-3-2019 by phakt]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Depends on where on the molecule you'd want to put it. It would be interesting if you tell us where and why you'd want a phenyl group at a specific
location.
For sure the amide can be phenylated in a Buchwald-Hartwig amination
And the alpha position of the pyrolidone also looks suitable for some kind of coupling reaction.
But in any case I doubt the most efficient route would use piracetam as a starting material and you're very likely to end up with too much heavy metal
content in the product for 'research purposes'.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
https://en.wikipedia.org/wiki/Phenylpiracetam
You could make it this way
1a - https://www.chem.wisc.edu/areas/reich/papers/Reich-1975-JACS...
1b - https://www.sciencedirect.com/science/article/pii/S004040390...
2.Reaction of the en-lactam with phenyl grignard in the presence of CuCl https://en.wikipedia.org/wiki/Reactions_of_organocopper_reag...
Since lactams(amides) are also carbonyl compounds ,albeit less reactive,they should also react similar to ketones to form en-lactams which should
further undergo michael addition to give phenylpiracetam
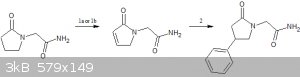
[Edited on 25-3-2019 by CuReUS]
|
|
|
rase
Harmless

Posts: 5
Registered: 25-3-2019
Member Is Offline
|
|
is pyrolidone an analog of phenylpiracetam?
looks like phenylpiracetam is not commercialized anymore in Russia.. what could be the alternatives to obtain?
is there a natural or a synthetic chemical with the same or similar properties of phenylpiracetam?
when the phenyl ring is added into piracetam what are the main changes on the piracetam properties?
Thank you
|
|
|
rase
Harmless

Posts: 5
Registered: 25-3-2019
Member Is Offline
|
|
BUMP.
can someone answer?
Thanks
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
No, its a precursor.
I think CuReUS's step 1 could be substituted with alpha halogenation of the ketone followed by E1 elimination. The question would be whether the
pyrrolidone ring is prone to hydrolosis in the somewhat harsh conditions required for the halogenation.
However, im not so sure enolation of amides is a very easy thing to do, the only way ive found requires low temperatures so maybe hes right.
Sufficiently advanced science is indistinguishable from madness.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
If the halogen is on the alpha carbon,when it leaves ,it will create a carbocation right next to the ketone.Since ketones are
electron withdrawing,the carbocation formed won't be stabilised and so the reaction won't happen.
For your idea to work,the halogen should be on the beta carbon  . The leaving
group in that case would be hydrogen as H+,and the corresponding carbanion formed would be stabilised by the electron withdrawing ketone . . The leaving
group in that case would be hydrogen as H+,and the corresponding carbanion formed would be stabilised by the electron withdrawing ketone .
[Edited on 28-4-2019 by CuReUS]
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Quote: Originally posted by CuReUS  |
For your idea to work,the halogen should be on the beta carbon  . The leaving
group in that case would be hydrogen as H+,and the corresponding carbanion formed would be stabilised by the electron withdrawing ketone . . The leaving
group in that case would be hydrogen as H+,and the corresponding carbanion formed would be stabilised by the electron withdrawing ketone .
[Edited on 28-4-2019 by CuReUS] |
Um, The H+ would be picked up by an alkoxide anion, the leaving group would be a halide which is picked up by an alkyli metal cation from the
potassium methoxide (alkali metal alkoxide).
The resulting electrons on the carbons would have only one thing left to do and that form a pi bond (double bond).
There is no proton on the carbonyl carbon but there is one at the beta position, therefore if the halide was at the alpha position then it would only
have one way for the elimination to go.
https://en.wikipedia.org/wiki/Elimination_reaction
In principle it is identical to the reaction you proposed however instead of a selenium oxide leaving group instead you have a halide leaving group,
the only advantage your paper suggests is lower temperature and milder conditions.
The resulting alkene can then undergo enantioselective addition.
Sufficiently advanced science is indistinguishable from madness.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Assured Fish  | Quote: Originally posted by CuReUS  |
For your idea to work,the halogen should be on the beta carbon  . The leaving
group in that case would be hydrogen as H+,and the corresponding carbanion formed would be stabilised by the electron withdrawing ketone . . The leaving
group in that case would be hydrogen as H+,and the corresponding carbanion formed would be stabilised by the electron withdrawing ketone .
[Edited on 28-4-2019 by CuReUS] |
Um, The H+ would be picked up by an alkoxide anion, the leaving group would be a halide which is picked up by an alkyli metal cation from the
potassium methoxide (alkali metal alkoxide). |
The leaving group by definition is obviously the halogen,but
the Hydrogen also has to leave(or get "picked up " as you call it) for the bond to form.What I am mainly trying to convey is that-
1.Carbocations CAN'T form next to electron withdrawing group
2.Carbanions CAN'T form next to electron donating groups
Can't form doesn't mean its impossible,but for most reactions,its doesn't happen
so putting a halogen on the alpha carbon is going to form a carbocation right next to the E withdrawing group,leading to destability and
consequently,a failed reaction.
| Quote: | | In principle it is identical to the reaction you proposed however instead of a selenium oxide leaving group instead you have a halide leaving group
|
please provide me a ref where an alpha haloketone is converted to an enone
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Alright fine, i got nothing.
Lesson learned, back to the books.
Sufficiently advanced science is indistinguishable from madness.
|
|
|
Optimum
Harmless

Posts: 20
Registered: 4-8-2019
Member Is Offline
|
|
can anyone tell a bit more about the actual procedure?
it would be better to synth phenylpiracetam from scratch or buy piracetam and use chemistry procedure to join phenyl to piracetam?
how could i do the second procedure?
Thanks
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Quote: Originally posted by Optimum  | can anyone tell a bit more about the actual procedure?
it would be better to synth phenylpiracetam from scratch or buy piracetam and use chemistry procedure to join phenyl to piracetam?
how could i do the second procedure?
Thanks |
The second step is a grignard reaction in the presence of a catalytic amount of a Cu(I) salt.
Cuprous chloride isn't the easiest to prepare mind you and grignard reaction are well, tricky and tedious with the amount of prep required.
This reaction and its yields will also suffer as a result of there being an amide present which will engage in side reactions.
The obvious solution is to keep the enone in excess.
If you don't have the know how to generate your own procedure from this knowledge alone then im sorry to tell you but you lack the knowledge to
achieve the desired results anyhow, this is a tricky reaction and work up.
Grignard reactions are famous though and there is a hell of a lot of literature on the topic, so perhaps there is where you should start.
Sufficiently advanced science is indistinguishable from madness.
|
|
|
Texium
|
Thread Moved
9-11-2019 at 16:14 |