| Pages:
1
2 |
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
How to concentrate azeotropic HNO3?
Think this wasn't asked before, so Im gonna ask this.
I have conc HNO3 which I think is ~68%, are there ways to concentrate it up to fuming concentrations without first turning it into nitrate salt?
[Edited on 190306 by fusso]
|
|
|
Cezium
Harmless

Posts: 39
Registered: 5-1-2015
Member Is Offline
Mood: No Mood
|
|
Destil with something that likes water more than HNO3 
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Will H2SO4 dehydrate it into NO2 & O2?
[Edited on 190306 by fusso]
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
Given that nitric acid is normally synthesised by reacting a nitrate salt with concentrated sulphuric acid to give fuming nitric acid in a high yield
(80%+), I would infer that conc. H2SO4 won't dehydrate it to the point of decomposition. My guess is that you're referring to
the equilibrium:
H2SO4 + HNO3 ⇌ H2O + HSO4- + NO2+
which is an equilibrium, and therefore is driven to the lefthand side by removal of HNO3. Therefore, concentrated sulphuric acid would
likely be a suitable drying agent.
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
98% sulphuric works beautifully.
I used to only be able to obtain 62% nitric acid back in the day and always just distilled it with double its volume of concentrated sulphuric acid to
produce red fuming HNO3.
If you want super high purity and clean water white fuming nitric just distill it again with a vacuum.
Be good, otherwise be good at it 
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
This not a dehydration but a decomposition, and no it will not dehydrate to an extent that would matter. Dehydration of HNO3 will give N2O5 which is
the dehydrated form of HNO3.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  |
This not a dehydration but a decomposition, and no it will not dehydrate to an extent that would matter. Dehydration of HNO3 will give N2O5 which is
the dehydrated form of HNO3. |
Won't N2O5 decomp into NO2+O2?
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
No, NO2 has a different and lower oxidation state than NO3- hence there has to be a reduction before it can go into NO2. Such as when copper metal is
added.
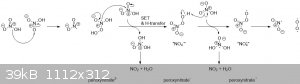
The following equation does happen, according to Wikipedia, but not to significant extent 4HNO3 --> 4 NO2 + 2 H2O + O2. That is it happens slowly
but over time enough to color your anhydrous nitric a red color. Or faster at higher temperatures.
Could anyone explain why this happens. I came up with a more or less mechanistic explanation but am not entirely convinced, especially not by the
disproportionation.
Perhaps:
2 nitrate ions disproportionate to one peroxy nitrate (2-) and one NO2.
Peroxynitrate(2-) then gets oxidized by HNO3 to peroxynitrate (1-), another NO2 and water.
Peroxynitrate(1-) then gets oxidized again to peroxynitrate radical, and another NO2 and water.
The peroxynitrate radical then dissociates into oxygen and NO2.
I'm sure AKJOER has some comments on this. But I doubt any of it, and my explanation included, will have any practical consequences.
Apart from, keep the temperature low so distill under reduced pressure or you will get RFNA.
[Edited on 6-3-2019 by Sigmatropic]
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I used equal volumes of 98% H2SO4 and 65% HNO3. This produced 1/2 the initial volume of conc HNO3. I never did a full analysis of the product, but the
density was 1.50 and produced RDX in the expected yield from direct nitration.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Distilling a mixture of HNO3 & H2SO4 sounds dangerous...any safer dehydrating agents? Does anhydrous nitrates/bisulphates work?
|
|
|
Cezium
Harmless

Posts: 39
Registered: 5-1-2015
Member Is Offline
Mood: No Mood
|
|
Nitrate + hydrogen sulfate salt destil works, but with lower yield and end up with hard to get out goo. Nacid+Sacid+lowP is proven and works.
HNO3+P4O10 also works, but...
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Quote: Originally posted by Cezium  | | Nitrate + hydrogen sulfate salt destil works, but with lower yield and end up with hard to get out goo. |
No,
that's not what I mean. I mean, do "azeo HNO3 + anhydrous nitrate salt" & "azeo HNO3 + anhydrous bisulphate salt" work?
[Edited on 190306 by fusso]
|
|
|
Cezium
Harmless

Posts: 39
Registered: 5-1-2015
Member Is Offline
Mood: No Mood
|
|
as I stated before, you have to find something that likes water more than HNO3 likes water, and of course doesnt react with stuff.
So, HNO3aq+XNO3solid NO nitrates dont do hydrates and dont dehydrate stuff.
HNO3aq+NaHSO4solid YES, hydrogen sulfate acts basically like conc. sulfuric acid, but as written before, you ends up with that goo in the flask.
TLDR: Man dont try to overcomplicate it and make your live simplier with proven methods that works. You are trying to reinvent the wheel.
[Edited on 6-3-2019 by Cezium]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Now, I assume you plan nothing terrible with this stuff.
So...... One of our members has already posted an elegant synthesis for this material.
Requires some vacuum to forestall thermal decomposition, but otherwise very do-able. I'll look for a link.
An hour passes...........
Man, that wasn't easy. Still on Youtube, but buried.
https://www.youtube.com/watch?v=DEEjNQN2dIQ
As for the use of gloves; regular gloves won't cut it. They tend to burst into flames, when concentrated nitric hits them.
https://www.youtube.com/watch?v=aBVdGGml6bU
[Edited on 7-3-2019 by zed]
[Edited on 7-3-2019 by zed]
[Edited on 7-3-2019 by zed]
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fusso  | | Distilling a mixture of HNO3 & H2SO4 sounds dangerous...any safer dehydrating agents? Does anhydrous nitrates/bisulphates work?
|
Everything we do has danger.
Chemistry wise this is just about the safest shit I can think of.
Distilling azeotropic Nitric from preferably fuming Sulfuric acid to get concentrated Nitric is how the world operates.
[Edited on 3/7/2019 by morganbw]
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
If you do not want to use sulfuric acid your best option is magnesium nitrate. It is a well recognized industrial route; you mix azeotropic nitric
acid with a 72 wt % solution of magnesium nitrate and distill. This yields concentrated nitric acid and a dilute magnesium nitrate solution. The
magnesium nitrate solution can then be re-concentrated and reused in future distillations. I am not entirely sure how you make a 72 wt % magnesium
nitrate solution given its solubility in water (106 g / 100 mL of water at 90 °C according to Wikipedia), but I think it involves melting the
hexahydrate, possibly under a vacuum. You would have to do some more research on that. A summary of the process can be found on page 34 of this pdf
which is a section from Ullmann's Encyclopedia of Industrial Chemistry.
http://www.ugr.es/~tep028/pqi/descargas/Industria%20quimica%...
[Edited on 7-3-2019 by Plunkett]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I have read that you can extract HNO3 from an aqueous solution with DCM with the exclusion of water. I am not sure what the partition coefficient
would be for this mixture, but I would start off with several small extractions with DCM and then weigh it to find the amount of extracted HNO3.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Loptr  | | I have read that you can extract HNO3 from an aqueous solution with DCM with the exclusion of water. I am not sure what the partition coefficient
would be for this mixture, but I would start off with several small extractions with DCM and then weigh it to find the amount of extracted HNO3.
|
I find that hard to believe.
Okay, I see it's been discussed here before, and only works in the presence of a large amount of sulphuric acid.
https://patents.google.com/patent/US3981975A/en
[Edited on 7-3-2019 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by Loptr  | | I have read that you can extract HNO3 from an aqueous solution with DCM with the exclusion of water. I am not sure what the partition coefficient
would be for this mixture, but I would start off with several small extractions with DCM and then weigh it to find the amount of extracted HNO3.
|
I find that hard to believe.
Okay, I see it's been discussed here before, and only works in the presence of a large amount of sulphuric acid.
|
You'll find that the time and effort required to raffinate the stuff hard to believe, as well...
But you could see it as arm exercise after a few hours of continuous, vigorous shaking!
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by fusso  | | Distilling a mixture of HNO3 & H2SO4 sounds dangerous...any safer dehydrating agents? Does anhydrous nitrates/bisulphates work?
|
This is the safest and easiest method available. You have only liquids and a fairly low boiling product. The
most dangerous thing is the product. Anhydrous HNO3 is MUCH nastier than normal azeotropic acid.
Using solid materials like NaHSO4 with NaNO3 or KNO3 is a pain in the ass. You get a hard like rock crust which transfers heat from your mantle to the
inner solids only with difficulty. You run a much higher risk of cracking your glass, you get very uneven heating, due to bad heat transfer and no
mixing.
Second best is using KNO3 or NaNO3 with conc. H2SO4. This combination gives fuming HNO3 as well and if you add sufficient H2SO4 you won't get too much
solid matter in your boiling flask.
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Quote: Originally posted by Plunkett  | If you do not want to use sulfuric acid your best option is magnesium nitrate. It is a well recognized industrial route; you mix azeotropic nitric
acid with a 72 wt % solution of magnesium nitrate and distill. This yields concentrated nitric acid and a dilute magnesium nitrate solution. The
magnesium nitrate solution can then be re-concentrated and reused in future distillations. I am not entirely sure how you make a 72 wt % magnesium
nitrate solution given its solubility in water (106 g / 100 mL of water at 90 °C according to Wikipedia), but I think it involves melting the
hexahydrate, possibly under a vacuum. You would have to do some more research on that. A summary of the process can be found on page 34 of this pdf
which is a section from Ullmann's Encyclopedia of Industrial Chemistry.
http://www.ugr.es/~tep028/pqi/descargas/Industria%20quimica%...
[Edited on 7-3-2019 by Plunkett] |
The 72% refers to partly dehydrated Mg nitrate which has a very strong affinity towards water. Thus it can be used instead of sulfuric acid in the
process. Debydration of Mg nitrate is performed under vaccuum and heating. Regular Mg nitrate in prill form is always accompanied by various huge
amounts of cristal water, part of which has to be removed from the salt before it gains enough affinity towards water to become usable in the nitric
acid concentration process.
Exact science is a figment of imagination.......
|
|
|
Vomaturge
Hazard to Others
  
Posts: 286
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
What Woelen said!
Even azeotropic nitric acid will burn skin more rapidly than sulfuric and even a first degree nitric burn will leave a yellow stain that won't wash off for days.
also, nitric acid emits more vapors (both HNO3 and NO2), so it's also an inhalation hazard.
I've heard concentrated sulfuric acid described as a "strong oxidizing agent." umm...
https://www.youtube.com/watch?v=aBVdGGml6bU
https://en.m.wikipedia.org/wiki/Aniline#Rocket_fuel
https://www.sciencemadness.org/whisper/viewthread.php?tid=63...
I've never heard of H2SO4 doing any of that except with chlorates (and nitrates of course  ). I'm not saying >95% nitric will ignite every flammable substance, but I think it oxidizes organic compounds more readily than
sulphuric can. ). I'm not saying >95% nitric will ignite every flammable substance, but I think it oxidizes organic compounds more readily than
sulphuric can.
Sulphuric acid is certainly very dangerous. It can destroy eyes, lungs (when in mist form) and even skin if not washed promptly. But any combination
of containment, ventilation, protective clothing and other safety measures that lets you handle fuming nitric acid will be plenty safe with
concentrated sulphuric acid.
[Edited on 7-3-2019 by Vomaturge]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | The 72% refers to partly dehydrated Mg nitrate which has a very strong affinity towards water. Thus it can be used instead of sulfuric acid in the
process. Debydration of Mg nitrate is performed under vaccuum and heating. |
According to the lit., Mg nitrate cannot be dehydrated as the salt decomposes at high temp..
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Hence the “partly dehydrated” part. One can not rid Mg nitrate of all the crystal water by heating.....that would lead to decomposition....but
enough to raise the affinity towards water above that of azeotropic nitric acid. Searcing for the MAGNAC process related patents should provide ample
information on the topic.
Exact science is a figment of imagination.......
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
You might break the 68 % azeotrope by freezing - the solid would be 55 %, and the eutectic freezing at -42 degrees would be 71 %.
The eutectic mother liquor would then no longer be azeotrope and you could therefore distil it... but at 71 %, it would be quite close to the 69 %
azeotrope and thus hard to separate. (Azeotrope composition generally does not stay constant with pressure. Which way does nitric acid azeotrope
composition change?)
You need to get over 91 %. Then you can separate the eutectic mother liquor (91 %, freezing at -66 degrees) from 100 % nitric acid.
[Edited on 8-3-2019 by chornedsnorkack]
|
|
|
| Pages:
1
2 |