mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Heumann indigo synthesis help
For quite a long time I'm struggling with synthesis of indigo dye. When I figured out that o benzaldehyde syntheses are nearly impossible for amateur
chemist and very impractical I prepared anthranilic acid and purchased chloroacetic acid but unfortunately I don't have access to any references. Can
somebody help me with paper describing synthesis of indigo in that way? Preferably heumann process as it seems to be the simplest (only naoh
chloroacetic acid and anthranilic are used)
Thanks
[Edited on 25-2-2019 by mackolol]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
| Quote: |
To a 2-L flask equipped with a condenser were added 137 g anthranilic acid (1 mol),94.5 g chloroacetic acid (1 mol), and 1 L water; the mixture was
heated for 3 h. On cooling,crystals of phenylglycine-o-carboxylic acid were separated out. These were filtered off and recrystallized from hot water
to give∼1000 g of such acid.The mixture of 1 eq. phenylglycine-o-carboxylic acid, 3 eq. NaOH, and 1 eq. water was heated at 235–265◦C in the
absence of air until the alkaline mass assumed an intense orange color, whereupon it was allowed to cool and subsequently dissolved in water. Passing
a stream of air through the aqueous solution resulted in the precipitation of indigo. No yield was given |
Heumann Indigo Process
https://doi.org/10.1002/9780470638859.conrr312
[Edited on 25-2-2019 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Thank you very much Waffles
|
|
|
NotBismuth
Harmless

Posts: 10
Registered: 24-2-2019
Location: Singapore
Member Is Offline
|
|
Seeing that Chloroacetic Acid is considered an extremely hazardous substance, would it be possible to substitute MCA with Glacial Acetic Acid?
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
No. If you draw out the reaction, you'll see why.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Another example of Heumann indigo synthesis

But i am interested in Pfleger's synthesis of indigo and i want to know this is possible to use Sodium Methoxide or ethoxide instead of Sodium amide?
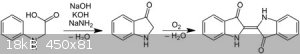
[Edited on 23-5-2019 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|