hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
Mandelic acid
As you know Mandelic acid prepared by below reaction

and also Mandelic acid is type of hydroxy acid
mandelamide perpared by reaction between mandelic acid+acetone +sulfuric acid and amonia
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3...
as you know acetamide prepared by heating ammonium acetate and heating ammonium mandelate should lead to mandelamide?!
also reaction betwen methyl or ethyl mandelate with ammonia too.
also i think this is possible from mandelonitrile?!
[Edited on 26-9-2010 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
Hexagon
Harmless

Posts: 45
Registered: 11-5-2010
Member Is Offline
Mood: Fanf*ckingtastic
|
|
AFAIK you can make mandelic acid from benzaldehyde and chloroform in the presence of a strong base like sodium hydroxide, an alcohol would be required
as a solvent. Check out chlorbutol synth. witch I think should be similar to this one.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
You certainly could use the ammonium salt but it might have a lower yield or side reactions. Amides can be formed directly from esters, and the
acetone condensation product looks like a reactive and easy to form ester.
Nitriles hydrolyze first into amides then into acids so you may be able to skip the extra step.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
Quote: Originally posted by Hexagon  | | AFAIK you can make mandelic acid from benzaldehyde and chloroform in the presence of a strong base like sodium hydroxide, an alcohol would be required
as a solvent. Check out chlorbutol synth. witch I think should be similar to this one. |
Reaction of benzaldehyde with chloroform in peresent of strong alkali lead to Trichloromethyl phenyl carbinol and i think hydrolyse of this componet
should lead to mandelyl chloride
http://pubs.acs.org/doi/abs/10.1021/ja01679a028
(Reaction of mandelyl chloride with ammonıa should lead to mandel amide directly)
Methyl mandelate should perpared by reaction of Mandelic acid and methanol but yield is low anybody has idea for get better yield(sure catalysis
needed.sulfuric acid is good choice?)?
also 'BORIC ACID CATALYZED AMIDE FORMATION FROM CARBOXYLIC ACIDS AND AMINES'
http://www.orgsyn.org/orgsyn/prep.asp?prep=v81p0262
I think ın this reaction i should use dry ammonia
Someone has idea for Resolution of Racemic mandelic acid?
[Edited on 28-9-2010 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
This is possible for Resolution of Racemic Mandelic acid by (R)-(+)-α-Methylbenzylamine or (S)-(-)-α-Methylbenzylamine
http://www.sciencemadness.org/talk/files.php?pid=152589&...
(R)-(+)-α-Methylbenzylamine(1-Phenethylamine) is expensive and it prepared by:
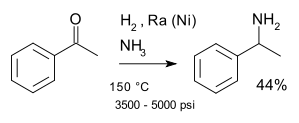
Anyone has better idea for making (R)-(+)-α-Methylbenzylamine or (s)-(-)-α-Methylbenzylamine?
Or better way for Resolution of DL-Mandelic acid?
Chemistry=Chem+ is+ Try
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Can't this be done by reducing the oxime with Fe/HCl ?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Isn't that structure formula wrong? The chiral bond shouldn't be at the benzene ring, with all the carbons being sp<sup>2</sup> hybridized
(planar configuration). Or am I missing something?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
No its fine, its just not conventional. Think of the bond originatning from the a-carbon of the alcohol and you'll see that thats all that matters.
Typically it would be the hydroxy group shown with undefined/racemic stereochemistry, as you should try and keep the carbon backbone as planar as
possible, if that makes sense.
|
|
|