hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
Oxidation of Styrene
Oxıdation of alkene with KMnO4 solution or Na2Cr2O7 produce alcohol or ketones or carboxylic acids İ wonder why oxidation of styrene by
Na2Cr2O7 or KMnO4 dont lead to Phenethyl alcohol or phenylacetic acid?
Also oxidation of alkene (carbon with two hydrogen) lead to CO2 and Carboxylic acid or alcohol
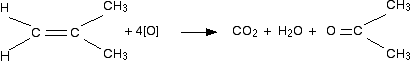
Oxidation of styrene with KMnO4 or Na2Cr2O7 lead to Benzoic acid and CO2 or another thing?
Also we know oxidation of ethyl benzene with Na2Cr2O7 lead to Phenylacetic acid but why not for styrene? carbon in alkene group in styren should be
more active than carbon in alkane group in ethyl benzene
[Edited on 19-9-2010 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
behemoth
Harmless

Posts: 17
Registered: 19-8-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hector2000  |
Oxidation of styrene with KMnO4 or Na2Cr2O7 lead to Benzoic acid and CO2 or another thing?
[Edited on 19-9-2010 by hector2000] |
That is correct. KMnO4 will cleave alkenes on the double bond giving ketones or aldehydes, the latter being further oxidized to the corresponding
carboxylic acids by excess KMnO4. There are procedures where styrenes are cleaved by aqueous KMnO4 to corresponding benzaldehydes, yields are not very
high though.
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
I think it would give you styrene oxide which could then give you acetophenone or perhaps benzaldehyde because of the double bond.
|
|
|
behemoth
Harmless

Posts: 17
Registered: 19-8-2009
Member Is Offline
Mood: No Mood
|
|
For styrene --> styrene oxide you will need oxidants containing a peroxo-group like oxone(R), peracids, peroxides or hypochlorite (bleach),
hypobromite.
The styrene oxide can then be opened in acidic aqueous solution to phenylethane(1,2)diol. Rearrangement to phenylacetic aldehyde is also possible.
I wonder if styrene would be cleaved to benzaldehyde according to patent US 808095 (see that thread) nicely, eg. without further oxidation to benzoic acid.
[Edited on 19-9-2010 by behemoth]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Styrene is readily oxidized, and rearranged into phenylacetic acid via the Willgerodt Reaction. There is even a microwave version of the reaction.
Since this method works, the reaction conditions are easy to produce, and the reagents are cheaply and readily acquired......Other oxidations aren't
usually attempted.
[Edited on 20-9-2010 by zed]
|
|
|
behemoth
Harmless

Posts: 17
Registered: 19-8-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zed  | Styrene is readily oxidized, and rearranged into phenylacetic acid via the Willgerodt Reaction. There is even a microwave version of the reaction.
Since this method works, the reaction conditions are easy to produce, and the reagents are cheaply and readily acquired......Other oxidations aren't
usually attempted.
[Edited on 20-9-2010 by zed] |
There is a thread regarding the Willgerodt reaction of styrene: http://www.sciencemadness.org/talk/viewthread.php?tid=6513
apparently the reaction conditions are not easy to produce (remember that chinese patent CN1110677) for the Willgerodt reaction itself. And reagents
like morpholine for the Kindler variation are not cheaply and readily acquired.
[Edited on 20-9-2010 by behemoth]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
That's a reasonably good link, and there are others.
Overall, I suppose it depends on what your definition of easy is.
Heating readily available Styrene, Isopropyl Alcohol, Sulfur, and Ammonium Hydroxide.........Under a few atmospheres of pressure, seems easy to me.
Now, making phenylacetic acid, and using it to synthesize illicit materials, may be an immoral pursuit......But, like many harmful, yet profitable
enterprises, it isn't difficult.
[Edited on 20-9-2010 by zed]
[Edited on 20-9-2010 by zed]
|
|
|
behemoth
Harmless

Posts: 17
Registered: 19-8-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zed  | | ...Heating readily available Styrene, Isopropyl Alcohol, Sulfur, and Ammonium Hydroxide.........Under a few atmospheres of pressure, seems easy to
me... |
Well, did you actually read the corresponding threat? No one could reproduce that chinese patent and I doubt that you would.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Perhaps no-one in that particular thread was successful. Experimenters elsewhere have reported success.
At any rate, since for the general overall well being of humanity, it is probably best that this reaction does not work, I retract my former
statements.
The Willgerodt Reaction doesn't work very well, and the reagents are impossible to acquire.
[Edited on 22-9-2010 by zed]
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
I wonder hydration of alkyne by Mercury salt and sulphuric acid as atalyst ,should lead to aldehyde or ketone but in phenylacetylene i think we will
have acetophenone not phenylacetaldehyde.why?!

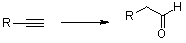
[Edited on 22-9-2010 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
In the case of the Styrene 1,2-diol, the reaction generally proceeds by the elimination of one of the -OH groups followed by rearrangement.
The Benzylic carbon, at position 1, forms (by far) the most stable ionic intermediate.
Therefore, the -OH at position 1, is the easiest to remove by dehydration.
Once the ball starts rolling down hill, a molecule of water is removed, a hydrogen atom shifts, and bingo! You end up with phenylacetaldehyde.
This is basis of the well known oxidation of propenyl benzenes, via peroxide-formic acid, to the epoxide, followed by hydrolysis to the diol, and then
dehydration/rearrangement to the benzyl-methyl-ketone ( phenyl-2-propanone).
[Edited on 22-9-2010 by zed]
[Edited on 22-9-2010 by zed]
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
Has anyone got any further references on this reaction?
It implies that styrene could be oxidized with iodine, mercury oxide and water? All of the compounds dissolve in water in minute quantities, should it
be enough to actually procceed the reaction?
| Quote: |
Styrene oxide can be prepared by the action of iodine, water, and mercuric oxide on styrene.1 The procedure described has been published
Hibbert and Burt, J. Am. Chem. Soc. 47, 2240 (1925). |
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Ether is involved, your reference has nothing to do with this, and the correct reference is Tiffeneau, Compt. rend. 140, 1595 (1905), who soon made
phenyl-2-propanol from it with MeMgI.
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
Quote: Originally posted by S.C. Wack  | | Ether is involved, your reference has nothing to do with this, and the correct reference is Tiffeneau, Compt. rend. 140, 1595 (1905), who soon made
phenyl-2-propanol from it with MeMgI. |
Hey hey hey, as long as we are randomly oxidizing styrene non-sequiter to the OP's intent....
Styrene + O3 = Benzaldehyde
Serious cautionary note: H2O2 is made in this reaction as a byproduct. H2O2 + Acetone (being their solvent used here), can generate
EXPLOSIVE peroxides - neutralize them before even thinking about distilling, and use testing strips to be safe~!
Better yet, read the paper written by professionals and heed their advice~!
I'm firing up my eBay Chinese Ozone generator as we speak~!
*Edit - credit for turning me onto this procedure goes to a science madness member by the name of Melgar, as he originally mused about in the big
benzaldehyde sticky thread.
Attachment: schiaffo2008 - Copy.pdf (138kB)
This file has been downloaded 421 times
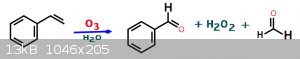
[Edited on 10-5-2020 by Johnny Windchimes]
~Incredibly profound and/or wise quote goes here~
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
I think I seen somewhere that styrene+copper oxide can lead to benzaldehyde
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
At least this concerns of it:
https://www.hindawi.com/journals/amse/2018/2716435/
How do they introduce the H2O2 into the reaction? The article states they used 30% H2O2 for the test.
And then there are the catalysts. Co-Ag catalyst, could it be doable to the amateur?
[Edited on 10-5-2020 by Refinery]
|
|
|