Methansaeuretier
Harmless

Posts: 45
Registered: 24-5-2010
Location: Europe
Member Is Offline
Mood: strongly alkaline
|
|
Toluhydrochinon formylation via reimer tiemann?
Hello there
I like to do a reimer tiemann formylation on toluhydrochinon to get 4-Methyl-2,5-Dihydroxybenzaldehyde in a ~100g scale. I already have the
hydrochinone, CHCl3 and NaOH/KOH. I'm planning to use a vacuum destillation for workup.
Has anyone instructions and/or any tricks for this reaction?
Another Idea is to make toluchinon via H2O2/I2 and monomethylation with MeOH a la https://sciencemadness.org/talk/viewthread.php?tid=9835 to give 3-methyl-4-methoxyphenol (or would it react to 2-methyl-4-methoxyphenol) and
formylation of this phenol, because p-methoxyphenol is known to give good yields on reimer tiemann formylation.
[Edited on 8-8-2010 by Methansaeuretier]
|
|
|
Methansaeuretier
Harmless

Posts: 45
Registered: 24-5-2010
Location: Europe
Member Is Offline
Mood: strongly alkaline
|
|
Today I started an experiment.
I dissolved 48 g toluhydrochinon (p.A.) in a solution of 150 g sodium hydroxide (techn.) in tap water (about 330ml total volume of a dark clear
solution) in an erlenmeyer flask (500 ml) and added while the solution was still warm (~50°C) 100 ml trichloromethane (p.S.) in small portions
(magnetic stirring) to prevent boilover. After some minutes tiny bubbles became visible (?what is it?). . Now, after 1h, the solution is still warm
to touch and foaming.
Made a TLC (Alugram Xtra Sil G/UV 254nm with toluene as eluent) against pure toluhydrochinon which indicates at least 2 Products. One product runs
very fast (nearly as fast a toluene itself) and the other one (seems to be the main product) runs slow. Pure toluhydrochinon doesn't move at all .
Both products are colorless on the layer in VIS-light.
Any ideas which products I got?
[Edited on 9-8-2010 by Methansaeuretier]
I let the mixture cool to room temperature. Some cristalls precipiated. I added some water to dissolve everything and than I added conc. HCl. Now I
have a dark but clear solution... tomorrow I will extract it (I don't know which solvent I have to use) and maybe try a bisulfit adduct.
[Edited on 9-8-2010 by Methansaeuretier]
Made some more TLC. See attachment
A=Pure toluhydrochinon
B=Reaction mix
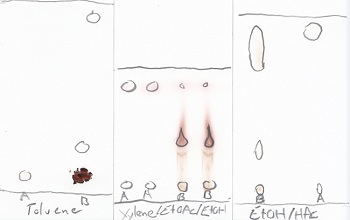
[Edited on 9-8-2010 by Methansaeuretier]
[Edited on 9-8-2010 by Methansaeuretier]
|
|
|