| Pages:
1
2 |
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Propyl-Iodide Preparation?
Once again I am a total chem noob(I feel the need to include this with every post). So as a novice, I'm looking for simple experiments to do!
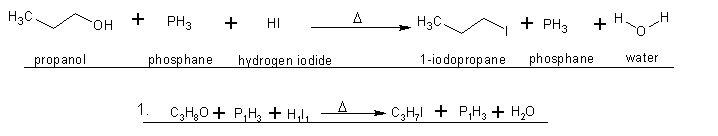
1. I was wondering if this 'write-up' looked right or if I mucked it up?(hopefully the attachment works okay). Edit - also, what kind of reaction is
this(by name), just for future knowledge ? ?
2. If the only by-product of this reaction is water, and according to wiki(not a great source I know), propyl-iodide is soluble in water("0.11 g/100
mL at 20 °C" - http://en.wikipedia.org/wiki/N-Propyl_iodide).
Hypothetically, couldn't you freeze the solution to maybe -17*C so the water would freeze, but the propyl iodide would remain a
liquid.(propyl-iodide's melting point = -101c : http://chemicalland21.com/industrialchem/organic/n-PROPYL%20...)?
Or is this an azeotropic relationship(apologies for lack of scientific vernacular)?
Thank you for your time you brilliant minds!
[Edited on 28-4-2010 by smaerd]
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
What the heck is the phosphine good for? Why do you wanna mess around with that?? 
Propanol would react with hydrogen iodide without the need for ridiculously toxic and self-igniting phosphine. The HI is often made in situ with
KI/NaI + H<sub>3</sub>PO<sub>4</sub>.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Well I for some reason was under the impression that PH3 was red phosphorus! YIKES! What a mistake that was...
so there's not even a need for a phosphorus catalyst?
"It has the chemical formula C3H7I and is prepared by heating n-propyl alcohol with iodine and phosphorus." - from wiki.
thanks for helping me out here jeesh, I mucked that up pretty good(go figure) lol.
hmmm...
[Edited on 28-4-2010 by smaerd]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Are you hell bent on halogenating with Iodine or would other halogens such as Bromine suffice? If you could I would no doubt go with the Bromo
derivative since its easier to aquire H2SO4 and NaBr to perform the reaction. I have posted a writeup on my Bromoethane synthesis using getto
equipment on here which should apply just as well here if not better due to the assumed higher Boiling point of Propylbromide over EtBr allowing more
time to react before being distilled from the reaction media.
PS: Was the propanol bought from a chem supplier or do you have a clean over the counter source for it? If so PM me please since I would like to have
to propanol without distilling assloads of floor stripper.
[Edited on 28-4-2010 by Sedit]
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
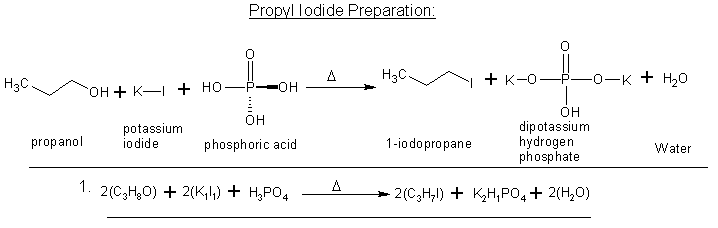
AH! I see -light-bulb-
so it'd be more like this?
propanol + potassium iodide + phosphoric acid --reflux--> propyl iodide + (still need to work out the products)
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Yes that is correct and you can find a nice writeup on here as well where someone did just that to yeild Methyl iodide.
Heres the Iodomethane writeup using H3PO4,
http://www.sciencemadness.org/talk/viewthread.php?tid=12475#...
[Edited on 28-4-2010 by Sedit]
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I'll have to read your bromoethane work-up, sounds interesting.
I have yet to find the the n-propanol(I have found sources that appear amateur friendly, but it looks pretty expensive), so this is kind of a in
future project. However, I could u2u the supplier, though it's kind of annecdotal(lack of better word) because I'd of never used them before. If you
were to use floor-stripper would you need to use fractional distillation or would simple distillation suffice?
Thanks so much for the information here, I've got a bit of rearranging to do with my idea in ACD's ChemSketch. I'll certainly post up the final
picture just to save a fellow noob the trouble, hehe. I want to make sure I have everything 100% on paper before I even consider it. Which makes
sci-mad a necessary resource  . .
[Edited on 28-4-2010 by smaerd]
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I know this is more of a "two-step" reaction, but does this look right by means of reactants and products?
I think I got it this time  . The only thing I'm a bit shakey on is the water
forming as the end result. Normally when Hydrogen gas(H2) and Oxygen form to make H2O isn't it a very violent reaction? . The only thing I'm a bit shakey on is the water
forming as the end result. Normally when Hydrogen gas(H2) and Oxygen form to make H2O isn't it a very violent reaction?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
More like
H3PO4 + KI <=> KH2PO4 + HI
HI + ROH => RI + H2O
The first H on H3PO4 is a fairly strong acid, and can release the halogen acids from their salts. The other 2 H are weaker acids, I doubt that the
2nd plays much of a role and certainly not the 3rd unless you are distilling HI or RI away from the reaction mix. Hmm .. the insolubility of alkyl
halides might drive the reaction enough to involve the 2nd hydrogen of the H3PO4, but I'm still doubtful.
H3PO4 works where H2SO4 doesn't because H3PO4 is not an oxidising acid at moderate temperatures such as can be tolerated by lab glassware.
In some cases you can use strong hydrochloric acid and a soluble iodide. HCl reacts noticeably slower with primary alcohols that does HI. The HCl and
say NaI give a mixture containing both HCl and HI, even though HI is the stronger acid enough forms that for some alcohols the insolubility of the
alkyl iodide keeps removing HI and drives the reaction to completion.
[Edited on 29-4-2010 by not_important]
|
|
|
Pomzazed
Hazard to Self
 
Posts: 57
Registered: 15-9-2008
Location: In th' Lab
Member Is Offline
Mood: Acylated
|
|
I doubt you'll get pure 1-halopropane.
acid attacks the alcohol, resulting in a primary carbocation (positive charge at terminal carbon); which in turn rearrange to the more stable
secondary carbocation (the charge at the center carbon) and then this combine with the nucleophile (halide; in this case iodide) resulting in
2-iodopropane.
Don't stare at me making fumes... I'm just experimenting with some gas...
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
So how would one theoretically purify the end-results of such a reaction then? simple distillation seems kind of unrealistic.
If HCL would work it'd be a real nice short-cut from ordering phosphoric acid. I just don't want to do anything too 'experimental'. I'm not afraid of
failure haha, I am just sure my first chemical creation will already be riddled with problems and bumps along the way as it is.
Another big question I have is how important of a role does purity of the acid play for these kinds of reactions? I know the acids have to be strong.
but like 70% strong, or reagent grade?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
As I just said in another thread : Org Syn collected vol 1 page 25.
Purification often is just washing and drying for the lower alcohols, for higher alcohols the water solubility is low enough that distillation might
be needed. Using HCl it is best to distill the product after wash&dry, as some RCl can be formed; or do the wash-up and dry, then add to a
solution of NaI in acetone to convert any RCl to RI, filter off NaCl(s), w&d.
Strength and purity are two different things. Impurities can cause side reactions, or end up in the product and perhaps require distillation to
remove. The strength needed is in part going to be determined by the alcohol being converted.
[Edited on 29-4-2010 by not_important]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Smaerd, how about learning the basics of chemistry before actually doing any experiment. For someone who thinks HI is iodine
and PH3 phosphorous ("phosphane"?) you seem too ambitious, especially by wanting to perform a reaction of which you don't have the slightest clue on
how it works. Your reluctance to read the reference not_important gave you, makes you appear quite irresponsible. Such an approach is often a good
recipe for disaster. You should start the opposite way, first learn the basics, then do some experiment based on what you learned and learn more from
the experience. Having an experience (or mishap) without having the basic tools to interpret it is of little to no use.
Pomzazed, the reaction is a simple SN2 substitution if primary alcohols are used. The acidic media is not for carbocation generation,
but just to "activate" the alcohol by protonation (thus making it enough electrophilic for the reaction to proceed at all). The reaction is thus:
R-OH + H<sup>+</sup> <=> R-OH<sub>2</sub><sup>+</sup>
R-OH<sub>2</sub><sup>+</sup> + I<sup>-</sup> => R-I + H<sub>2</sub>O
(H<sup>+</sup> is just a formal way of describing an acid catalyst without actually describing the whole proton transfer process -
otherwise "naked" protons as such do not exist in solutions)
There is no HI involved (if you check the pKa of HI you can see that it is present only in very tiny amounts). This is also consistent with the
observation that the ease of formation follows R-I > R-Br > R-Cl as the nucleophilicity of halides in protic solvents drops analogously:
I<sup>-</sup> > Br<sup>-</sup> > Cl<sup>-</sup>. If the reaction would be SN1 then the rate of alkyl halide
formation would be independent from the halide used. An interesting demonstration of it being an SN2 reaction would therefore be to use HCl instead of
H3PO4 as acid catalyst and analyse the R-I vs. R-Cl ratio in the product. Being an SN2 reaction, there should be nearly no R-Cl formed on primary
alcohols. With tertiary alcohols which follow the SN1 mechanism, there should form a considerable mixture of both t-alkyl halides.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
"how about learning the basics of chemistry before actually doing any experiment."
Nicodem that's what I'm doing right now  . Like I said above, I'm not doing
anything until I know 100% what I'm doing. I'm not there yet at all, obviously. That's why I'm here, to pick up information, and find out more of what
I need to know. You're prolly thinking "hit the books", and your right, I should, and I will! Right after I get back from class. . Like I said above, I'm not doing
anything until I know 100% what I'm doing. I'm not there yet at all, obviously. That's why I'm here, to pick up information, and find out more of what
I need to know. You're prolly thinking "hit the books", and your right, I should, and I will! Right after I get back from class.
Also I've been reading and researching every resource given to me. Please don't think I haven't! Whether or not I understand it all, is another story.
However, he gave me that reference at the very end of this thread and I fell asleep before checking back here:
http://www.orgsyn.org/orgsyn/default.asp?dbname=orgsyn&d...
It looks like an incredibly valuable resource for what I'm trying to understand. Thanks not_important  . .
I know I'm new here and new to chemistry in general, but I'm not without any commonsense. I'm a new chemistry major, I understand the scientific
method, and I'd never do anything I'm unsure of. Hence why I haven't even considered aquiring anything for this idea, cuz I just don't know it(which
in turn is also why I am here)! Sorry for such a long drawn out post of little value.
[Edited on 29-4-2010 by smaerd]
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
On the link provided by not_important, it shows

and it says it'll work for n-pr, assumedly that's n-propanol.
Now that is for the ROH-Br. couldn't the same be done but with I instead of Br?
|
|
|
Pomzazed
Hazard to Self
 
Posts: 57
Registered: 15-9-2008
Location: In th' Lab
Member Is Offline
Mood: Acylated
|
|
@Nicodem
Yes I know that. But that's why i said i "doubt" he will get "pure" 1-halopropane. I did get fraction of a rearranged isomer the attemp of
pentanol->halopentane from fractional distillation, though pentyl is larger thus having more steric hindrance than a propyl. I did not say he wont
get the product, but "pure" product.
@smaerd
The equation shown above use the different reagent (phosphorous bromide) and have kinda different mechanism.
Don't stare at me making fumes... I'm just experimenting with some gas...
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
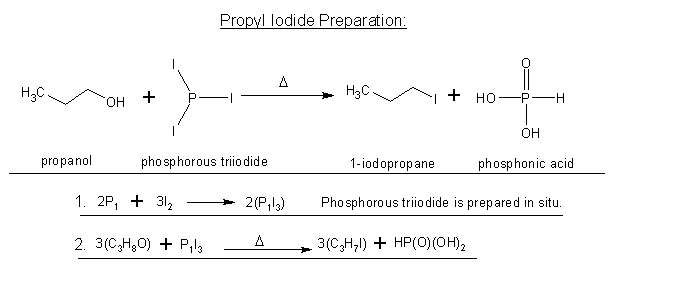
well I talked to someone who is a biochem major(years ahead of me) and he looked over it and said it would work. So I don't know what to think now...
Wiki's statement also agrees that this would work. So who should I trust, you guys, or my friend and wiki?
PI3 would be the reagent instead of PBr3(obviously).
[Edited on 29-4-2010 by smaerd]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Nobody said it wouldn't work, just that it was a different mechanism; Using PI3 is slightly different to using KI and H3PO4 , though it would
work, as would other reactions like the Appel reaction, using CX4 and PPh3:
R-OH + CX4 + PPh3 => R-X + CHX3 + Ph3P=O (IIRC)
where X = Cl, Br, I.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Hopefully, I finally got this right.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Smaerd, that is different reaction from the one discussed upthread and it is (still) not clear if you are aware of this.
Quote: Originally posted by Pomzazed  | @Nicodem
Yes I know that. But that's why i said i "doubt" he will get "pure" 1-halopropane. I did get fraction of a rearranged isomer the attemp of
pentanol->halopentane from fractional distillation, though pentyl is larger thus having more steric hindrance than a propyl. I did not say he wont
get the product, but "pure" product. |
Are you sure? Have you analysed that fraction? By what means?
I don't have much first hand experience in preparing alkyl halides by acid catalysed halogenation of alcohols (except for isopropyl bromide and
isobutyl bromide). I did however once made 1-bromo-3-phenylpropane from 3-phenylpropanol using 48% aq. HBr (the product is otherwise dirt cheap, but I
did this out of curiosity more than need). This was a biphasic reaction so it is not exactly the same. Anyway, at room temperature there was no
reaction (by HPLC). It did go well at reflux though. There were no unaccounted side products on the HPLC chromatogram and the product was pure by 1H
NMR (no isomers as far as I remember).
Also, keep in mind that the potential presence of isomeric alkyl halides in the nucleophilic acid catalysed halogenation of primary alkyl alcohols is
not proof enough that the reaction proceeds (or partially proceed) via SN1 substitution (that is via carbocations - with the exception of electron
rich benzylic or allylic alcohols). The origin of the sec-alkyl halides could still be accounted by implying a more probable elimination-addition
pathway: E2 elimination of the primary alcohol to the terminal alkene and electrophilic addition of HX to this which gives a sec-alkyl halide. To
complicate things even further, some alkenes give regioisomeric side products upon electrophilic addition of HX resulting from the hydride shifts in
the intermediate secondary carbocations. But primary carbocations from simple primary alcohols are highly unlikely and require usually more than just
H3PO4 or HX (once again with the exception of benzylic and allylic alcohols, etc.). These acids are certainly no superacids.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Pomzazed
Hazard to Self
 
Posts: 57
Registered: 15-9-2008
Location: In th' Lab
Member Is Offline
Mood: Acylated
|
|
| Quote: |
Also, keep in mind that the potential presence of isomeric alkyl halides in the nucleophilic acid catalysed halogenation of primary alkyl alcohols is
not proof enough that the reaction proceeds (or partially proceed) via SN1 substitution (that is via carbocations - with the exception of electron
rich benzylic or allylic alcohols). The origin of the sec-alkyl halides could still be accounted by implying a more probable elimination-addition
pathway: E2 elimination of the primary alcohol to the terminal alkene and electrophilic addition of HX to this which gives a sec-alkyl halide.
|
Agreed here.
| Quote: |
Have you analysed that fraction? By what means? |
The fraction (minor) from distilling after the reflux gives a colorless liquid out at 115.5C plus it has the smell of haloalkane. So it's likeliy to
be the 2-bromopentane. Though there is no further analysis of this fraction because it was such a small amount comparing to the scale, so it was just
then disposed away. (not the product i want anyway). The next major fraction comes out next at 129.5C which is 1-bromopentane.
Don't stare at me making fumes... I'm just experimenting with some gas...
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
Can this method be extended to do
phosphoric acid + KBr + EtOH -> EtBr + KH2PO4 + H2O
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Smaerd, if you want to do some experiment on preparative synthesis, please give us a list of chemicals you have and we'll give you a proposal and
guide you, first trough the learning process and then trough the practical aspect. I think this would be a much wiser approach for someone in your
situation and you would certainly learn a lot more.
| Quote: | | Quote: |
Have you analysed that fraction? By what means? |
The fraction (minor) from distilling after the reflux gives a colorless liquid out at 115.5C plus it has the smell of haloalkane. So it's likeliy to
be the 2-bromopentane. Though there is no further analysis of this fraction because it was such a small amount comparing to the scale, so it was just
then disposed away. (not the product i want anyway). The next major fraction comes out next at 129.5C which is 1-bromopentane.
|
Maybe if you used H2SO4 and did not add enough water. Depends on the conditions you used, but already in the first place I would be sceptical that
this fraction was truly 2-bromopentane without proper characterisation. Besides, your experience can not be generalized to all similar reactions, even
those using iodides with H3PO4 on other primary alcohols. If this was a serious and common problem then the Org. Synth. entry would have
surely mentioned it somewhere among the so many examples given (some of which use quite harsh HBr/H2SO4(aq) mixtures with heating).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I have been distracted by finals this week so I haven't had much time to finish my work-up. However, finals are over, and I am back to researching,
and writing. I have none of the chemicals required at this point in time, so this is very much a dream still. I figure tomorrow I'll put a few hours
into it and hopefully I'll have the theoretical all down on paper/electronic document for your review/consultation.
Thanks a bunch guys  . It means a lot to me that I can get some guidance and some
extracurricular studying done before my next semester. . It means a lot to me that I can get some guidance and some
extracurricular studying done before my next semester.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Alright, I think this is everything. I rounded some decimals so I know it's off by a little bit(according to the law of conservation of mass), and I
wouldn't do it with a batch as big as this obviously haha.
Does everything look right to you guys?

|
|
|
| Pages:
1
2 |