andre178
Hazard to Self
 
Posts: 61
Registered: 11-12-2009
Location: San Diego, CA
Member Is Offline
Mood: pacified
|
|
stepwise synthesis of alkane macrocycle
hello everybody, I have a few questions, and possibly a validity check on the following reactions that I am trying to do in order to synthesize a
octomalonyl based macrocycle. I postulated using a template ligand but I don't think that's a viable solution. This is all in dry dichloromethane
(under Argon).
I begin with monobenzyl malonate (purchased from Aldrich, I would love to be able to make this for cheaper-but asymmetric yield isn't good.) this has
the benzyl ether protecting group (colored in purple).
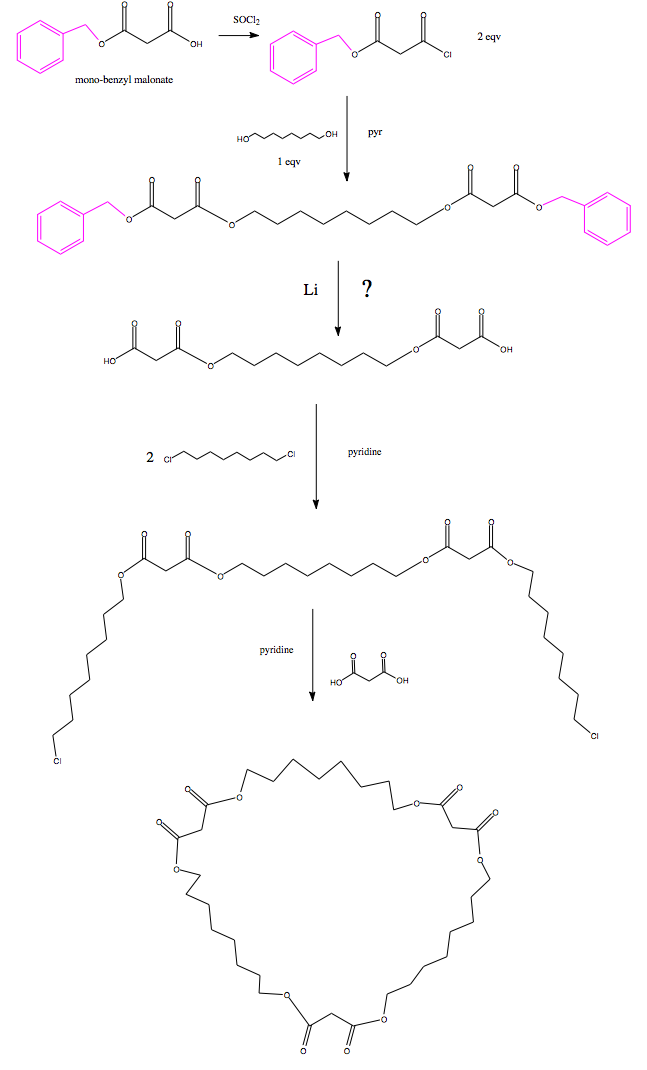
-------------------------------------
My first question: does anyone see any theoretical problems with what I am trying to achieve?
Second question: how can I remove the protecting groups without reducing/hydrogenating the carbonyls/esters. I was thinking about lithium based
catalysis but I am not sure. [I have never used P.G.'s before.]
Thanks for any input, even if its not right, I need more second opinions on this.
Andre
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I'm curious how you're preventing the 2,2 adduct. Purification? Excess of the alkyl chloride? And there might be something about kinetics or
availability preventing significant amounts of higher polymers (including polyester per se), like conducting the condensation in dilute
solution.
I guess pyridine doesn't react with the alkyl chloride? Seems funny as the alkyl chloride feels acidic, but I realize that's only when it's got a
protic solvent around to misbehave. Now, it'll certainly do a great job sucking away the HCl formed in condensation.
I'm afraid I can't be of much more help. I do look forward to seeing the results. (MP, TLC, IR and NMR I hope?)
Tim
|
|
|
andre178
Hazard to Self
 
Posts: 61
Registered: 11-12-2009
Location: San Diego, CA
Member Is Offline
Mood: pacified
|
|
I don't see which 2,2 adduct you're referring to, please specify, you could be right.
The pyridine acts as a base catalyst, it seems to do a fine job esterifying; all this is done in dry methylene chloride
.... and yes I might end up doing it in high dilution in order to get it to not run out of control.
purification wise I'm thinking at the end run through a column with CH2Cl to flush out pyridine salts etc, then do 90:10 CH2Cl2/EtOAc
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
That would not, strictly speaking, be classed as a true "macrocyclic alkane", because of the 6 lactone ( -C-O-C(=O)-C- ) linkages which occur in 3
pairs, instead of the ring consisting only of C atoms. A compound like that is likely to have properties similar to the macrocyclic "crown ethers",
used to chelate the cations of highly electropositive metals (which favor oxy-ligands) so as to enable them to be dissolved in non-polar organic
solvents.
True macrocyclic alkanes or alkenes, containing more than 6 C atoms in a ring, are very difficult to obtain, especially pure and free from polymers
(produced by intermolecular condensation), because of the difficulty in obtaining intramolecular ring closure involving carbons that are large
molecular distances apart on the same molecule, and this also applies to macrocyclic ethers and lactones and lactams etc. However, several
macrocyclic natural products are known. The most well-known such ones are atropine and cocaine, containing 7-membered all-C rings. Another one is
civetone, or cis-9-Cycloheptadecen-1-one, with a 17-carbon ring containing one double bond and having a keto group, in the "musk" obtained from the
scent glands of the African civet cat for perfumery, although thankfully the stuff can now be made synthetically from a fatty acid found in oil-palm
oil.
Attempts to obtain macrocyclic alkanes or alkenes or ethers or lactones sometimes result in the production of small amounts of catenanes, i.e.
supramolecular dimers or polymers in which large macrocyclic rings are linked as in a chain. It appears that rings containing at least about 19 atoms
are needed to obtain any of this chain-linking.
[Edited on 23-4-10 by JohnWW]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I see a problem, the synthesis is completely unselective for your final product, you will get everything, namely polymers and even larger cycles.
The step in question will likely go just fine with hydrogenation using a Pd/C catalyst. Of note, platinum catalysts will not work well for this. The
benzyl moiety will be reduced to toluene.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
What smuv says is not only true for the last step (reaction of the dichloro-derivative with malonic acid), but also from compound 3 to 4 - you'd have
to use a very large excess of 1,8 dichlorooctane to prevent cyclisation with 1 molecule of 1,8 dichlorooctane rather than producing
the compound 4 which you desire (reaction with 2 molecules of 1,8 dichlorooctane). Likewise, you'd have to use less than an equivalent of malonic acid
to get from 4 to 5, and dropwise addition, to prevent polymer formation or larger cycles.
Out of curiosity, how readily does the reaction proceed with a chloroalkane and a carboxylic acid, forming ester and HCl (or Pyridine-HCl)? What are
the condiitions for this?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
That's not a benzyl ether protecting group, it's an ester. There's nothing much to differentiate it from the alkyl chain when you attempt to cleave it
off. The use of such long chains is going to seriously inhibit proper cyclicization. Using short diols that can later be functionalized and ring
opened are much better bets.
Why so big? That size ring is extremely hard to close, whereas smaller rings (11-15 membered for example) are vastly easier to make (>50% yield by
some methods).
Are you dead set on the malonic acid? The acidity of it's alpha hydrogens would make the diol synthons I mentioned earlier harder to functionalize
after ring-closure.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
If you can redesign your molecule so that the open chain form has an alcohol at one end and an acid at the other, lactonisation of said molecule can
be had very easily using Yamaguchi conditions. Ring size isn't really an issue as I recall it being used to selectively form a 42 member ring, as
opposed to the 41 membered one that could have formed (obviously a case of stereoelectronics but still very impressive!)
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
@UC - benzyl esters undergo hydrogenolysis with pd/c preferentially to benzyl ethers. This deprotection strategy is widely known for both esters and
ethers.
ex. http://pubs.acs.org/doi/abs/10.1021/jo01105a031
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Interesting, thanks.
A good move to use might be to utilize heterocyclics incorporating a sulfur to bridge two carbons and pinch off 6 or 7 carbons, significantly
shrinking the ring that needs to be closed. Sulfur can be reductively stripped from molecules by rayney nickel afterward.
[Edited on 4-25-10 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
andre178
Hazard to Self
 
Posts: 61
Registered: 11-12-2009
Location: San Diego, CA
Member Is Offline
Mood: pacified
|
|
good information to keep me from pulling my hair out.
The rate of R-CO-X/R'-OH is slow, but it is decent using milimolar quantities. I am indeed performing this under high dilution, dropwise addition (8
hours for 500 ml solvent). It helps to prevent too many secondary by products.
This is all done in CH2Cl2 for a reason using non protic components besides the reactants. The deprotection step is done in an alcohol from what I've
seen.
====>>
Pd-C deprotection on Organic Chemistry Online
I am looking into the Yamaguchi esterification procedure, it looks very mild and a sure way to cyclize the straight chain. The problem now is the
stepwise addition to make a straight chain. The difficulty remains because of the repeating malonic acid+octanol units.
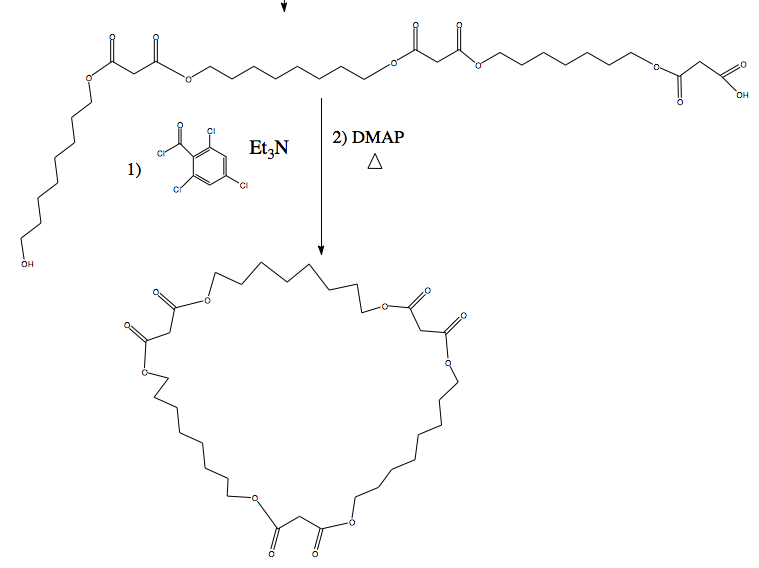
The sulphur containing heterocycle could also be an interesting alternative, I'm just thinking that it might react with the carbonyl groups from the
rest of the molecule.
Also, JohnWW, you are abs correct, it's not an alkane macrocycle, however it is pretty non polar compared to most heterocycles out there, which is why
I chose that title; but I will change that. I've been scanning through a few select books on the subject and it's rare to find more non polar chains.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
What I meant by sulfur-containing cycles is to use something like 2,7-bis(hydroxymethyl)thiepane or
2-(2-hydroxyethyl)-6-hydroxymethyltetrahydrothiopyran as a 1,8-octanediol synthon. The resulting primary chain is then 5 or 6 units long respectively
versus 8 when using the unfunctionalized diol.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
andre178
Hazard to Self
 
Posts: 61
Registered: 11-12-2009
Location: San Diego, CA
Member Is Offline
Mood: pacified
|
|
I like the heterocycle idea very much, hypothetically using a triphenylmethane template, followed by malonyl chloride reaction and then raney nickel
hydrogenation.
The heterocycle with the bis methanols would be difficult to synthesize, (2,7-dibromooctane-1,8-diol).
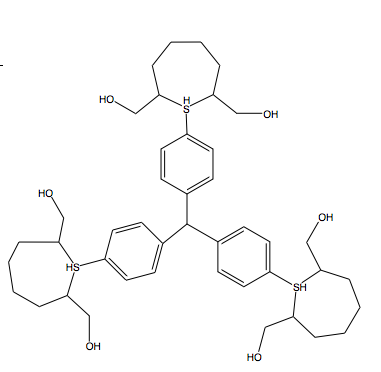
|
|
|