Costanza9
Harmless

Posts: 4
Registered: 11-4-2010
Member Is Offline
Mood: No Mood
|
|
Determining the configuration at a stereocenter
Hey, i'm kind of confused on determining the configuration of the stereocenter labeled 6 in this molecule.

Would the hydrogen at the stereocenter 6 be on the wedge coming out of the plane?
[Edited on 11-4-2010 by Costanza9]
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
Absolutely. It's the only bond not shown - the other three are all defined (into or in the plane of the "page") - so the remaining bond (out of the
page) must bear the hydrogen. Likewise at C7, the hydrogen must be on the dashed bond, going into the page.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
That molecule is reserpine, a tranquilizing drug. I understand that in most countries it can only be prescribed by specialist psychiatrists, and it is
used to tranquilize psychotically and criminally insane patients in secure mental hospitals.
There is a better illustration of its structure, showing full configurations at all its 7 stereogenic centers (including the tertiary-amine N), in
Morrison & Boyd's Organic Chemistry, 3rd (1973, page 1004) and 6th (1992/2002) Editions. I have uploaded both editions to my rapidshare.com
premium account, and posted the links for them in the References section. On the center labeled 6 above, the -OCH3 appears to go into the plane of
the page, and the H goes above the plane.
|
|
|
Costanza9
Harmless

Posts: 4
Registered: 11-4-2010
Member Is Offline
Mood: No Mood
|
|
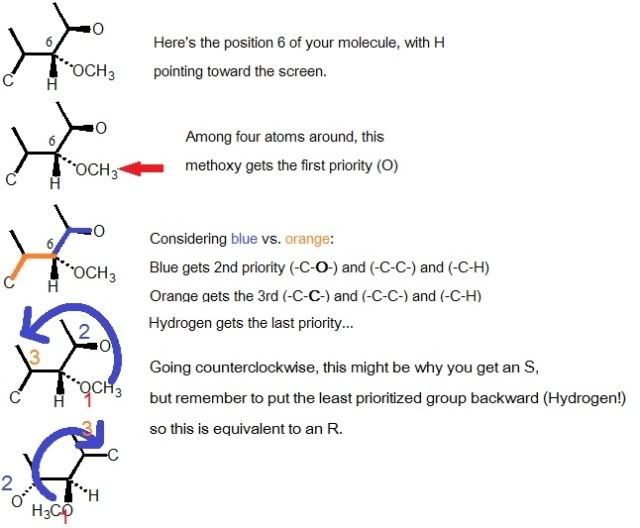
I keep getting an S configuration for the stereocenter labeled 6, when It should be R.
|
|
|
Pomzazed
Hazard to Self
 
Posts: 57
Registered: 15-9-2008
Location: In th' Lab
Member Is Offline
Mood: Acylated
|
|
Don't stare at me making fumes... I'm just experimenting with some gas...
|
|
|
Costanza9
Harmless

Posts: 4
Registered: 11-4-2010
Member Is Offline
Mood: No Mood
|
|
Wow, that is awesome. Thank you so much!
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
It's what I've been explaining to my students lately - the group with the lowest priority MUST be on the dashed bond, or you'll get the wrong answer.
But, since there are only two possible answers, you can just switch your answer to the correct one!
|
|
|
chemoleo
|
Thread Moved
12-4-2010 at 16:39 |