mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Mystery of the yellow benzophenone
I acquired some benzophenone on ebay that is supposed to be used for absorbing UV light in cosmetic products.
Unfortunately the powder is light yellow instead of white. It doesn't dissolve in water and floats on the top. Adding NaOH pellets forms a piss yellow
solution and the powder sinks to the bottom or forms a suspension on stirring.
I tried contacting the seller but he avoids my questions. I think it is not pure benzophenone but one of its derivatives. It looks like its useless
for the sodium-benzophenone method.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
I believe dioxybenzone is also used in sunscreens, is yellow, and insoluble in H2O.
E-bay?
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Have you looked at its IR, UV, and NMR spectra? If it is yellow, due to a strong absorbance in the blue to near-ultraviolet region, its UV spectrum
should differ considerably from that of benzophenone, which being white and used as an UV absorber in cosmetics,, should have no appreciable visible
(longer than about 400 nm) absorbance at all, but absorb strongly in the ultraviolet (shorter than about 400 nm).
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
So me and mr.crow did a group order from ebay, but since the photo was white and it stated 'benzophenone' when it isn't, we managed to get a refund.
I found the melting point to be 68degC, and only obtained a IR spectra. At this point, dioxybenzone is the best match based on what information I do have (little)
Although it looks strange compared to other IR spectras that I've seen, it isn't due to noise. At first I thought it was because I had too much sample
in the KBr pellet, but diluting it even further only clarified it slightly. Does anyone have access to IR spectra databases, and could pull out the
spectra for dioxybenzone?
Photo of the spectra below (don't have a scanner)
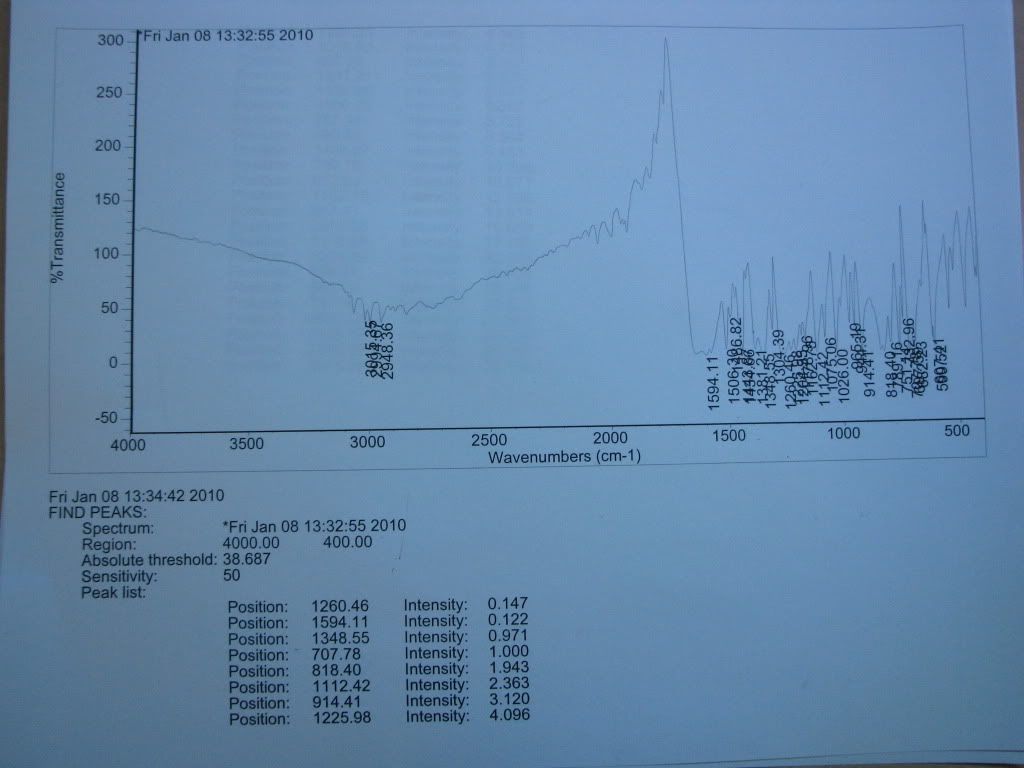
(Sorry if its a little too large)
I can try later to get a UV-vis spectra in ethanol, the machine was in use at the time.
Another thing comes to mind, what on earth can we do with a pound of this stuff?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aonomus  | | Although it looks strange compared to other IR spectras that I've seen, it isn't due to noise. At first I thought it was because I had too much sample
in the KBr pellet, but diluting it even further only clarified it slightly. Does anyone have access to IR spectra databases, and could pull out the
spectra for dioxybenzone? |
You still did not dilute it enough.
The IR spectra can be found at SDBS or Sigma.
| Quote: | | Another thing comes to mind, what on earth can we do with a pound of this stuff? |
Amateur chemical experiments perhaps?
Surely you have some imagination.
Edit: Corrected the defective SDBS link.
[Edited on 11/1/2010 by Nicodem]
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
The IR spectra peaks vaguely match the ones from Sigma, so it doesn't confirm or deny anything. 300% transmission???
One idea is to oxidize the ketone to produce phenolic compounds. I don't think this is possible, there is only one carbon atom between the phenols and
it looks very stable.
Another idea is to try and create some sort of antihistamine with it, but thats crazy.
One thing that looks like it would work is a Clemmensen reduction to make the diphenylmethane. Skip the mercury of course.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Instead of being goal oriented, think like a beginner and use it to as a model substrate to learn practical methods:
- Bromination, iodination or nitration to 2,2'-dihydroxy-4-methoxy-5-halo/nitro-benzophenone or polychlorination to
2,2'-dihydroxy-4-methoxy-3,5,3',5'-tetrachloro-benzophenone.
- Reduction of the carbonyl with Na2S2O4 or NaBH4 to give the corresponding benzhydryl alcohol.
- Baeyer-Villiger oxidation to 2-hydroxy-4-methoxyphenyl salicylate.
- Oximation to the corresponding benzophenone oxime and the reduction to the benzhydryl amine.
- Alkylation of the phenolic groups with home made alkyl bromides.
- Acetylation of the phenolic groups with acetanhydride.
... just to list a few.
Learning organic synthesis methods by actual experiments is more precious than just make something because it has some use or property.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Chlorination will be too much like dioxin or PCBs 
The Baeyer-Villiger reaction is the one I was looking for, or the Dakin reaction. It would be interesting to see which products are produced,
salicylic acid or catechol (or both) and separating them.
Alkylation also seems possible.
The problem with using a strange substrate is the product will be a new chemical without any information on it.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|