| Pages:
1
2 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Preparation of Pyridine
Preparation of Pyridine (Ordenblitz)
by Magpie
12/14/09
Introduction
This procedure is for the preparation of pyridine by decarboxylation of nicotinic acid using a copper chromite catalyst. It is based on the method
presented by Ordenblitz in the ScienceMadness forum.

Procedure
The copper chromite is produced following the procedure outlined in Organic Syntheses, Coll. Vol. 2, p.142 (1943); Vol. 19, p.31 (1939), except that
the crude material obtained after calcining of the barium copper ammonium chromate is used “as is,” ie, the subsequent acid washings are omitted.
The catalyst material obtained is reported to be: Cr2CuO4•CuO•BaCrO4.
CAUTION
Due caution is to be exercised when handling the reagents and product. With the exception of the nicotinic acid all exhibit some degree of toxicity.
This is especially true of those compounds containing hexavalent chromium. Soluble copper and barium compounds are also poisonous.
A. Preparation of Copper Chromite
Cu(NO3)2 + Ba(NO3)2 + (NH4)2Cr2O7 ---> complex chromates ---(ignition)---> Cr2CuO4•CuO•BaCrO4 + gases (likely NOx + NH3 + H2O)
The preparation of this catalyst will be per OrgSyn’s “Copper Chromite Catalyst,” using 1/3 quantities and omitting the acetic acid washes.
Add 8.67 g of barium nitrate to 224 mL water and warm to 70°C. Stir until dissolution is complete.
Add 72.67g of Cu(NO3)2•3H2O to 42.7mL of water to form a solution 4M. Add this to the barium nitrate solution. Hold at 70°C.
Mix 42g of ammonium dichromate with 200mL water. Add this to 50mL of 28% aqueous ammonia. It will turn from orange to yellow. Pour this solution
into the 70°C Ba/Cu nitrate solution in a thin stream. A brick red precipitate will form immediately, which is copper barium ammonium chromate.
Catch the precipitate with a 7cm Buchner filter. Spread the precipitate on a dinner plate and dry at 110°C.
Place a muffle furnace in the hood and set at 400°C. Break up the dried precipitate into chunks. Place the precipitate in covered crucibles and
ignite them for 1 hour in the muffle furnace at 400°C. The precipitate will turn blue-black. Expected yield is about 45g.
B. Preparation of Pyridine
Prepare for simple distillation using a 100mL RBF as pot, a 25mL receiver, and an 80-watt heating mantle as shown in the photograph below. Pre-dry
the glassware in an oven. Use cool water in the condenser and wrap an insulated blanket over the upper part of the pot.
Intimately mix 24g of nicotinic acid with 12g of the copper chromite catalyst using a mortar and pestle. Place this mixture in the 100mL RBF and
assemble the apparatus in a simple distillation configuration. Bring up the heat slowly starting at 50% power, rising ultimately to 65%. There will
be a fair amount of refluxing which may be beneficial in washing down any sublimed nicotinic acid from the pot walls. The bp of pyridine is 115°C.
The pyridine will come over in spurts of about 10 drops every minute at the peak of production. This will slow as the run progresses. The product
volume will indicate a yield very near the theoretical 13.1 ml. Any contamination of the product is likely to be a small amount of water. A picture
of the distillation set up is shown below:
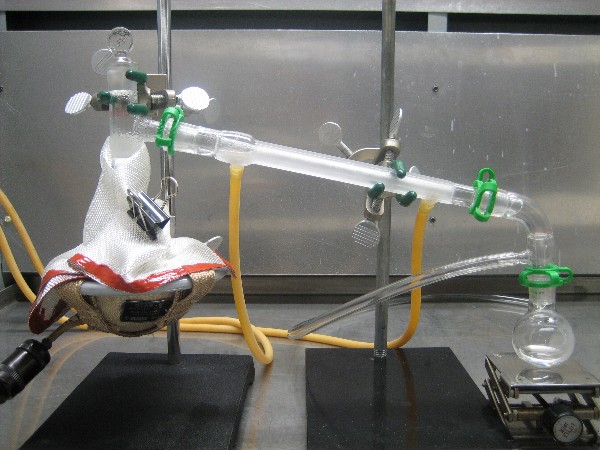
Below is a picture of the powder following the run. You can see the sublimated nicotinic acid as a hoarfrost on the surface. This powder broke up very
easily and there was no damage to the RBF.

Results and Discussion
Based on the nicotinic acid charge of 0.16 moles, and a sp gr for pyridine of 0.983, the theoretical yield is 13.1 mL. Something near this is to be
expected.
Notes
Nicotinic acid is sold OTC as niacin and niacin was used for this preparation. Copper nitrate and barium nitrate were made from homemade nitric acid
and pottery grade materials. The ammonium dichromate was technical grade.
The product likely contains some water. No attempt to purify it or obtain an indication of purity was made. Its smell was distinctly that of
pyridine.
This is the full scale method of ordenblitz as posted in ScienceMadness.org. It worked very well. At first a small scale method was tried but problems
with sublimation of the nicotinic acid, etc, resulted in failure.
References
1. Ordenblitz: http://www.sciencemadness.org/talk/viewthread.php?tid=9717&a...
2. The ordenblitz method is modified from the paper by T. A. Scott: http://www.biochemj.org/bj/102/0087/1020087.pdf as originally offered by LSD25 on ScienceMadness.org
[Edited on 15-12-2009 by Magpie]
[Edited on 15-12-2009 by Magpie]
[Edited on 15-12-2009 by Magpie]
[Edited on 15-12-2009 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Nice prep magpie. I think as well as actually producing pyridine (which you should purify  ), it is remarkable that the residue can be removed from the flask with ease! Its just a shame that niacin is so expensive. I do think that
with sufficient copper chromite "catalyst" the reaction can be scaled up somewhat so long as the niacin precursor can be economically obtained. ), it is remarkable that the residue can be removed from the flask with ease! Its just a shame that niacin is so expensive. I do think that
with sufficient copper chromite "catalyst" the reaction can be scaled up somewhat so long as the niacin precursor can be economically obtained.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
This is so much better than the typical dreary post - what the forum promises but seldom delivers - to my mind anyway
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Nicotinic acid should be obtainable from the cleavage of nicotine, extracted from tobacco plants. I have recently obtained some tobacco seeds, with
the help of another forum member, and intend growing them to extract nicotine, although primarily for use as an insecticide (although only in
situations where it cannot harm non-target species like bees).
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
About a month ago, I synthesized some pyridine by the same method after reading the results of Magpie's and Ordenblitz's experiments with nicotinic
acid and copper chromite. The nicotinic acid was produced by the hydrolysis of nicotinamide with 6M hydrochloric acid. Dilute sodium hydroxide
solution was added to the nicotinic acid hydrochloric acid solution until, the isoelectric point was reached with a pH meter. A large amount of
crystals formed and the solution was heated to boiling and water was added until everything just dissolved. After cooling overnight, the
recrystallized nicotinic acid was filtered and air dried for several days. 27.3 g of nicotinic aicd and 14g of commercial copper chromite were mixed
together in a 250 mL round bottomed flask. A distillation head and receiver flask was attached. The flask was heated with a mantle until no more
liquid distilled. The crude pyridine was light yellow in color and was redistilled, collecting between 97-110 C, a clear liquid that had the
characteristic foul smell that pyridine has. 11.2g of pyridine was obtained for a percentage yield of 64%. The pyridine was then converted into
pyridinium hydrobromide perbromide by dissolving it in 24 mL of azeotropic hydrobromic acid. The solution of pyridine hydrobromide was chilled in an
ice bath and 8.0 mL of bromine was added in small portions with swirling. An orange solid which soon turned into a red solid precipitated. The
solution was cooled and the crude product was filtered. The product was then recrystallized from glacial acetic acid. The long orange needle shaped
crystals were filtered off and pressed to remove most of the adhearing acetic acid. The product was then dried at room temperature. the slightly moist
product weighed 31g. This compound can be used to brominate alkenes; it is great for small scale experiments since it is easy to measure small
quantities. Next time I make pyridine I plan to make some pyridinium chlorochromate PCC.
[Edited on 15-12-2009 by benzylchloride1]
Amateur NMR spectroscopist
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I would like to make PCC also. Do you have a procedure for making it? Seems like I saw one some place but can't find it now.
[Edited on 15-12-2009 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
PYRIDINIUM CHLOROCHROMATE
@Magpie
The enclosed paper from TL Vol 16, No. 31(1975) pp 2647-2650, gives details of preparation of this compound and also its use in reduction of alcohols
to carbonyls.
Hope this helps.
gsd
Attachment: 2647-2650.pdf (205kB)
This file has been downloaded 5208 times
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by gsd  |
The enclosed paper from TL Vol 16, No. 31(1975) pp 2647-2650, gives details of preparation of this compound and also its use in reduction of alcohols
to carbonyls.
gsd |
Thanks gsd! Very nice!
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
unome2
Harmless

Posts: 14
Registered: 21-5-2009
Member Is Offline
Mood: No Mood
|
|
There is another paper on the destructive distillation of the Cu. Nicotinate, which is also supposed to give pyridine
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
| Quote: | | There is another paper on the destructive distillation of the Cu. Nicotinate, which is also supposed to give pyridine |
Here is an excerpt from that paper:
| Quote: | At - 278 and 280 o C a fast weight loss is observed in Cu(nic), and
Cu(isonic), respectively. The overall weight loss is in agreement with the
stoichiometry
5 Cu(NC5H4CO2)2(s) + 5 Cu(s) + 10 CO2(g) + 8 NC5H5(g) + N2(g) + 10 C(s)
Carbon dioxide was detected as the main gaseous product through the
formation of BaCO, upon bubbling the released gases in aqueous Ba(OH),,
and this was confirmed by IR spectroscopy of the evolved gases. Pyridine is
also formed and partially condenses; it can be identified both in the gas and
condensed liquid through IR spectra. |
Reference: Thermochimica Acta, 1989, vol. 138, p. 233 - 240
[Edited on 16-9-2010 by madscientist]
I weep at the sight of flaming acetic anhydride.
|
|
|
Polverone
Now celebrating 21 years of madness
|
Thread Split
24-11-2011 at 00:44 |
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
You might be able to avoid messing around with copper chromite by using this method: http://www.organic-chemistry.org/abstracts/lit2/764.shtm
Looks easy.
[Edited on 5-2-2012 by 497]
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Nice Magpie . . .will try soon!
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you. I don't know if you have ever smelled pyridine before. It's distinctive...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
No . . .
are other catalysts possible other than the copper chromite (I have no barium salts and very little dichromate and copper nitrate) for this reaction,
short again of the one in the above link?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Hexavalent  |
are other catalysts possible other than the copper chromite (I have no barium salts and very little dichromate and copper nitrate) for this reaction,
short again of the one in the above link? |
Did you look at the suggestions of others in this thread? However, I can't speak for them.
It is often the case that the biggest challenge of a synthesis for the home chemist is the gathering or making of the necessary reagents and
precursors.
"If wishes were horses, beggars would ride." 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I have to agree . . .I look at big syntheses that I would enjoy, only to find I am missing one central reagent
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Like tert-butyl lithium? 
I was considering this synthesis for awhile, but I can't remember why..hmm
[Edited on 3-2-2012 by AirCowPeaCock]
BOLD
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Hexavalent, if you read the <a href="http://www.orgsyn.org/orgsyn/prep.asp?prep=cv2p0142"><em>Org. Syn.</em> article</a>
carefully, you'll notice that the catalyst can be prepared without barium. However, the catalyst would be more susceptible to sulfate poisoning and
reduction.
[Edited on 3/3/12 by bfesser]
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Thanks for that reference, I will be sure to read that.
AirCow - I highly doubt one could prepare t-butyl lithium in an amateur setting . . .handling it is one thing, making it is quite another.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
I was referring to this; Hexavalent:
http://www.sciencemadness.org/talk/viewthread.php?tid=18480
BOLD
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
you can do the same thing by heating to 320 C with 1/3 CaO and 2/3 NaOH aka soda lime, with nicotinic acid.
it's very hard to decarboxylate that ortho acid but it can be done.
[Edited on 11-7-2012 by jon]
Give me librium or give me meth!
Patrick Henry....
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by jon  | you can do the same thing by heating to 320 C with 1/3 CaO and 2/3 NaOH aka soda lime, with nicotinic acid.
it's very hard to decarboxylate that ortho acid but it can be done. |
And the reference is ...
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Improved Preparation of Pyridine
Preparation of Copper Chromite Decarboxylation Catalyst
The orgsyn prep1 of this material utilizes barium nitrate, which reportedly protects against sulfate poisoning of the catalyst. This is
an issue during hydrogenation, but not likely during decarboxylation. Copper nitrate is necessary, since using the sulfate would precipitate BaSO4. I
have omitted the barium nitrate and replaced the copper nitrate with the more readily available (for most) copper sulfate.
14.95g (0.059mol, all I had) of ammonium dichromate is placed in a ~200ml beaker and 35ml of distilled water and 50ml of 10% aqueous ammonia is added
(or equivalent of stronger aqueous ammonia and distilled water). The orange solid dissolves with stirring to give a yellow solution of ammonium
chromate. In a seperate beaker, 29.48g (0.118mol, 2eq) of Copper sulfate pentahydrate is dissolved in 200ml of distilled water. Warming may be
necessary to speed the dissolution.

With stirring, the chromate solution is poured into the copper sulfate solution resulting in an orangey-brown finely granular precipitate that slowly
settles out of the olive colored supernatant.

The product is vacuum filtered and pressed as dry as possible, though it still retains a fair amount of liquid. It is dried in an evaporating dish of
known mass at 110C until constant weight, providing 16.98g of chalky, discolored product with patches of dried salts that were wicked to the surface
during drying.

If you have a furnace capable of maintaining 400C, place this material in a loosely covered crucible and heat for 1 hour instead. A
hot plate was turned on and adjusted so that the surface temperature was a stable 400C as verified by an IR thermometer. For the author's Corning
PC-351, this was about 1/3 of the way between 5 and 6. The evaporating dish is placed on the hotplate and covered with a watch glass. After a few
minutes, a small amount of condensation begins to collect on the watchglass. Soon, steam containing a small amount of ammonia begins to jet out of the
evaporating dish.

This vigorous reaction slows down soon and reveals that most of the solid has been converted into a friable black material with patches of whitish
salts. Material higher up in the evaporating dish may not have "ignited" and the product is broken up and stirred with a spatula ocasionally, pushing
orange pieces into the center. The heating is maintained for 1 hour, after which there should be no residual orange material.

The material is crushed readily into a fine black powder in the evaporating dish using a stopper or small RBF as a "pestle."
This crude catalyst can likely be used as is, but I chose to wash it as per the orgsyn procedure to remove residual salts.
The powder is suspended with stirring in 100ml of 10% acetic acid. After 10 minutes, the stirring is stopped, and the solid allowed to settle for ~15
minutes after which roughly 2/3 of the deep green supernatant can be removed by careful decantation or use of a pipette. An additional 100ml of 10%
acetic acid was added and stirred for 10 minutes more. The lighter, but still very green supernatant was removed as completely as possible after
settling for 10 minutes.
To the remaining liquid and catalyst is added 100ml of distilled water, stirred into a suspension for a minute or two and allowed to settle for ~10
minutes, and decanted. This is repeated another time. The solid is then suction filtered and washed with two 50ml portions of distilled water.

This solid is dried until constant weight, giving 11.72g of copper chromite catalyst as a fine, dusty black powder.
Decarboxylation of Nicotinic Acid to Yield Pyridine
While the thread author, magpie uses 50wt% of copper chromite catalyst to perform the decarboxylation, I suspected that this was a massive excess.
Indeed, an orgsyn procedure for the preparation of imidazole2 by decarboxylation of imidazole-4,5-dicarboxylic acid utilizes only 0.5g of
copper chromite per ~0.3mol of acid. An attempt was thus made to use a similar catalyst loading for nicotinic acid.
In a beaker, 61.56g (0.5mol) of nicotinic acid, obtained as niacin from purebulk.com was mixed with 1.00g of copper chromite catalyst to a uniform,
light gray powder.

The mixture was loaded into a 500ml 1-neck RBF along with a few boiling stones for good measure (very likely unnecessary, the catalyst has plenty of
surface area), and set up for simple distillation. When initially heated, the nicotinic acid amusingly begins to sublime upwards into the mass of
powder, creating a gap underneath it.

This mass eventually collapses onto the bottom of the flask and melts into a bubbling pool of black liquid. Use of a free flame on the outside of the
flask near the top of the mass of powder will speed up this process. Nicotinic acid will begin to sublime onto the flask walls.

Soon, droplets of pyridine begin to condense in the 3-way adapter at a stillhead temperature of 105-110C. If the heating is increased to bring the
stillhead temperature to the boiling point of pyridine (115C), a considerable amount of subliming nicotinic acid will start to be washed into the
receiving flask. Keeping the temperature lower allows considerable reflux of pyridine to keep most nicotinic acid out of the stillhead. The pyridine
that is carried over at a moderate rate is most likely due to CO2 flow from the ongoing decarboxylation.

The above image shows the stillhead at 115C (too hot). Whitish material can be seen entering the condenser which is sublimed nicotinic acid being
carried by pyridine. This condensate should normally remain clear.
When considerable nicotinic acid builds up on the flask walls, application of a free flame to the outside of the flask will melt it and cause it to
fall/drip back into the reaction mixture where the catalyst can act on it. The volume of this melt decreases as the reaction proceeds and product
collects in the receiving flask. When there is nearly no material left in the distillation flask and pyridine slows/stops distilling over, heating is
stopped. The nicotinic acid on the flask walls may be yellowish at this point. A small amount of tar and catalyst sits at the bottom of the flask.

The product is known to contain some nicotinic acid, and so it is returned to the reaction flask along with a stirbar. The mixture is distilled
normally, with refluxing pyridine washing any and all nicotinic acid down into the flask's bottom. The product passes over as a colorless liquid at a
stillhead temp of 115C.

The product weighs 34.10g and is a clear colorless liquid with a characteristic and unpleasant smell. This is an 86% yield from nicotinic
acid. For those keeping track, the crude product before redistillation weighed about 36.1g. The 2g discrepancy roughly correlates to 500ml of
pyridine vapor that remained in the boiling flask. Using a chaser solvent or decreasing the flask size (or increasing batch size) would thus slightly
improve this yield up to ~90%. Evaporation of a drop of the distilled product on a clean glass surface left no appreciable residue, whereas a drop of
the crude material left a white film of nicotinic acid. No further purification was carried out, but allowing the material to stand over solid KOH (or
better yet, CaH2) and redistilling would remove any traces of water that may be present. The tar in the flask is most easily removed with NaOH
solution.

References:
1) Organic Syntheses, Coll. Vol. 2, p.142 (1943); Vol. 19, p.31 (1939).
2) Organic Syntheses, Coll. Vol. 3, p.471 (1955); Vol. 22, p.65 (1942).
[Edited on 10-25-13 by UnintentionalChaos]
[Edited on 10-26-13 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
Wow UC, that is a beautifully detailed and well illustrated write up. Great job and very interesting, in particular the changes you made and the
clear statement of yield.
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
Yes .Well done UC. Best illustrated write up I've seen on SM for quite some time.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
| Pages:
1
2 |