| Pages:
1
2 |
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
Holiday Experiments
Not much activity here lately - so I thought I would post some of the chemistry stuff I did over the holiday.
I made KNO3 from NH4NO3 and KCl. I am quite familiar with KNO3 from my high school pyro days, but had not used any since graduating from college.
Ended up with the familiar white crystals. When dry, I tested by mixing about 1/2 gram with an equal amount of powdered sugar. Lit with a butane
torch and confirmed it burned fairly well (not as good as black powder, but it did confirm I had KNO3).
I also made KClO3/4(?) using electrolysis. I used a small plastic container with about 150ml of saturated NaCl solution. For the anode, I used two
carbon rods connected in parallel from a "heavy duty" lantern battery. For the cathode I used a piece of steel plate from a gas grill
igniter. I ran around 2 amps through the solution for about 48 hours. About half the rods got eaten away in that time. I filtered out the graphite
and then mixed with KCl and chilled. Got around 11g of precipitate (theoretical is 30g or more, but the temperature of the solution was too cold for
efficiency - it was sitting outside on my 3rd floor patio). I mixed about 0.5 grams with an equal amount of powdered sugar and it burned almost in a
flash when lit with a butane torch. I then made another small batch of the KClO3/sugar and dropped concentrated H2SO4 (drain cleaner) on it. This
made a splattering/frying sound for about a second, then ignited and burned rapidly.
I tried making sodium acetate using baking soda and vinegar, but did not have any luck making the supersaturated solution that crystalizes all at once
when you drop in a crystal of sodium acetate. I neutralized about 1 pint of distilled white vinegar with baking soda. I wanted excess carbonate so
that when I boiled the solution away there would be no vinegar smell. But I found I still had NaHCO3 left and this started to precipitate when about
20% of the water was left. So I added more vinegar, this time checking pH until I had a slightly acidic solution. I was able to get down to less
than 10% of the water before crystals started forming. But when I let it cool, I had a somewhat thick yellow solution that would not crystalize
anything no matter how many crystals I dropped in. I tried heating it some more and crystals immediately formed. It was almost as if crystals were
forming more at higher temperatures than at lower temperatures.
I obtained Mg ribbon. Although at one time I had powdered magnesium, I have never had Mg ribbon before. I was surprised that this burned rather
poorly. It took 5 to 10 seconds to ignite with a butane torch. I had the ribbon sticking out with the end sitting on a scrap steel plate. The Mg
kept going out even before it reached the steel, and even heating it with a torch would not get it to burn in contact with the steel. The result
looked like I ended up with mostly oxide. I had read that Mg produces mainly the nitride when burning in air. I added a couple drops of water to the
ash and did not smell any NH3.
I also obtained some calcium metal in 0.2g chunks. I had read conflicting reports about its reactivity. The WebElements (www.webelements.com) says that calcium will react slowly with water, beginning to liberate hydrogen after "an hour or so". Other places
have described the reaction as vigerous enough to set the H2 on fire. When I dropped the calcium into water, I immediately got hydrogen bubbles at a
fairly brisk rate. The Ca initially sank but eventually floated due to all the bubbles once it got small enough. It took only a minute or two for
all the Ca to be used up. The MSDS with the Ca said it is extremely flamable. I tried burning a 0.2g piece with butane torch, expecting a bright red
flame, but was disapointed. I heated it to a dull red glow, and still it did not burn. I heated some more until an orange glow. I took the torch
away and the orange glow got brighter, so I assume it was burning. It looked about the color of burning coals. But I never saw any red, and the
reaction soon stopped. After it cooled, I added water. Again, I had read that Ca forms mostly the nitride when burning in air. I expected to smell
NH3 but did not. It did bubble a bit, but I think this was probably H from remaining unreacted Ca.
Any ideas for interesting things (not necessary pyro) to do with the Mg and Ca? I was thinking the Ca would probably react directly with S to produce
CaS. Would this reaction likely be violent (Ca is a chunk, not a powder).
Hodges
|
|
|
Mumbles
Hazard to Others
  
Posts: 436
Registered: 12-3-2003
Location: US
Member Is Offline
Mood: Procrastinating
|
|
Can't really think of anything off the top of my head you could do with either of those things. I did think I would share what I accomplished
over the break though.
I made Nitrocellulose. I used a nitrate/acid method and got pretty good results. something like 75% yield. I don't know whats normal, but it
seems good enough. It burns very fast with a nice poof. I actually made two batches. One with cotton pads, and another with paper towel. I used
equal amounts of both and let them nitrate for the same amount of time.
The paper towel product is pure crap. Its burns slower, with residue and smoke, and for some reason it turned brown. Its a very odd product. It
dissolves in acetone, but when I add water I don't get a precipitate, or a very little one. I'll probably just keep burning it for fun
until it runs out.
I also made some octanitrosucrose. It's sticky. Well some is. I have floating blobs that are very sticky at temps above about 10, and this
fine gritty powder thats not sticky(higher nitrated probably). Its still drying so I don't know yields, but it looks not too bad.
I also disposed of some Iron Picrate that had formed. It wasn't much, just a couple crystals. I also disposed of my entire batch of iron oxide
just for safety.
Other than that I did a bit of partying. I also went through and cleaned up my lab. It was begining to be a bit of a mess. I cleaned all my
glassware too. I found out about this homeschool store a few towns over. I'm checking that out tommorow. I have broken a few
"beakers" lately, and my supply is running low. I've always wanted real beakers I can heat to a decent temperature. Well, I hope
everyone had a nice safe holiday.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I, unfortunatly, was not around untill 2am this morning but while i was away have planned on making SnCl4, sodium(by electrolysis and thermit
reactions), CHCl3 and a few other things. All I have actually done since I got back is KMno4 + glycerin because I found a little jar of KMnO4 in a
drugstore during holidaying.
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
If I were you, I'd save both metals in a noble gas atmosphere if possible and under lock and key, for making Grignard reagents in the future. I
wouldn't be too thrilled if someone decided to burn it.
Probably the most exciting thing I did during these holidays was to burn some steel wool. I started out using Brillo, but used up most of my MEK
dissolving away the soap. I dumped the saturated solutions on concrete, leaving a stubborn bright white stain from impurities (it was nothing more
than nail polish remover). When I actually fluffed it up and burned it, I made sure I wasn't going to get hit by the sparks so I gloved and
goggled up. When I looked closely, it was like red to yellow worms were crawling through the wire rather than points. I didn't set off any
fireworks, but instead got some boric acid, steel wool without soap in it, and a bit of titanium.
The other chemistry related thing I did was to make chlorine to gas some algae and bugs. I had a lot of MnO<sub>2</sub> left over from
gutting out lantern batteries and HCl, so I mixed a lot of both together making sure I didn't get even a whiff. Too bad I did after about 30
minutes when I wasn't paying attention for a bit. It burned my nose some and that's when I quit. I considered trying steel wool and
chlorine together, but it was much to windy to capture the latter in sufficient quantities and I wasn't about to try it indoors even in my shed.
Not too exciting, eh?
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
| Quote: | Originally posted by blip
I considered trying steel wool and chlorine together, but it was much to windy to capture the latter in sufficient quantities and I wasn't about
to try it indoors even in my shed. |
If you use fine steel wool, it will ignite spontaneously in chlorine, producing an orange cloud of smoke. If it is not fine enough, you can ignite it
first and then lower into the chlorine for the same orange smoke. Do this outdoors of course!
Hodges
|
|
|
overseer
Harmless

Posts: 24
Registered: 31-12-2003
Member Is Offline
Mood: Relaxed
|
|
>Any ideas for interesting things (not necessary pyro) to do with the Mg and Ca?
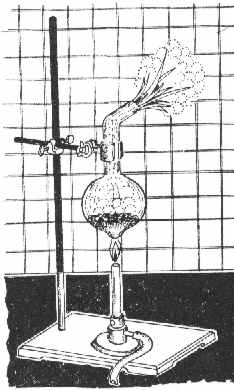
You could try obtaining some Si through a simple, yet *dangerous* reduction procedure involving Mg:
SiO2 + 2Mg -> Si + 2MgO
You'll need 3 g SiO2 (quartz, sand), 2.4 g Mg-dust and some HCl 1:1. Since You have the ribbon, You'd have to do some shredding. In
addition, You'd need: a larger test tube made of high-temp glass or a flask similar to the one in the Figure, a 250 ml beaker, a porcelain
grinding bowl, some standard lab stuff and very good *dark* goggles.
Procedure:
A mixture of 3 g finely powdered SiO2 and 2.4 g Mg is put into the flask and mixed thoroughly with a glass rod. The flask is then mounted as shown in
the Figure, and heated with a Bunsen burner. Initially, the whole mixture is heated, but later only the bottom of the flask, until the mixture begins
to glow (sometimes it takes 2-3 mins). Upon ignition, the flame is removed, since the reaction proceeds vigorously, liberating much heat and with a
strong flash of light.
Obtaining amorphous silicon in this manner creates also some Mg2Si, through a reaction between reduced Si and free Mg, at the high reaction
temperature. After cooling, the reaction mixture is poured onto a suitable piece of paper (usually the flask has to be broken in the process) and is
then added slowly, with mixing, into a beaker with HCl 1:1. After the MgO and Mg2Si have dissolved completely (which is indicated by the cessation of
sparking, popping and development of white fumes over the acid surface), the dark residue in the bottom is amorphous Si. The reaction between MgO,
Mg2Si and HCl involve the creation of silanes, that immediately ignite on contact with air and burn giving off SiO2 and H2O.
Finally, the liquid is poured off, and the residue washed several times with dilute HCl and finally with water. The amorphous Si dust is dried on 100
deg. Celsius. Do not heat it above the said temp. since it reacts on the surface with air oxygen, creating SiO2 again. It can be dissolved in HF acid
with some HNO3 added.
This procedure is from an introductory inorganic preparative chemistry textbook. Again, it is very dangerous, so You're doing it at Your own
risk! Don't do it unless You have enough experience. From my experience, many types of glass are prone to cracking when cooled from high temps,
and some high-temp type glasses can crack with small pieces flying all around after even cooling in air, back to a room temperature, if it was red hot
(goes esp. for thick wall vessels). The procedure should be done under the hood, so You should at least do it outdoors.
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
Sand and Mg
It is interesting that the Mg reacts with SiO2. I have seen in MSDS for Mg that one way to extinguish a Mg fire is with sand. Apparently if you dump
sand over burning Mg, the Mg goes out. Perhaps the silicon reduction only occurs if both the Mg and Si are finely powdered and well mixed.
I also know that Mg fires should not be faught using water, because hydrogen is produced. However, apparently an excess of water will still put out a
magnesium fire (though of course possibly producing enough H2 to be dangerous). I took a short piece of Mg ribbon and put one end in water and lit
the other end. It went out upon reaching the water. I also tried burning a coiled piece of Mg ribbon that had just a drop of water in the center.
It produced a finely divided ash (MgO I'm sure) which got blown into the air (probably by steam) and it took a couple minutes before the last bit
of MgO "snow" fell out of the air because the ash was so fine and light. I did get a slight but definite NH3 smell that time(from magnesium
nitride produced by burning in air, and the water).
Hodges
|
|
|
Al Koholic
Hazard to Self
 
Posts: 98
Registered: 2-12-2002
Member Is Offline
Mood: Seeking ligand
|
|
Well, since my distillation thermometer broke a couple weeks back I wasn't able to proceed with a vacuum distillation of nitric acid from
KNO3/H2SO4, and I decided to wait on making another batch of chloroform because I couldn't monitor the purification properly. I was also going
to distill chloropicrin but....
All I managed to get around to doing was to oxidize naphthalene with a 5:1 KMnO4/naph ratio in boiling solution for 4 hours. I was faced with some
interesting and forseen problems during the procedure like how to get the damn naphthalene back down into the flask from the condensation on the walls
of the reflux condenser. I made a nice steam generator from a flask, stopper, and glass tubing that actually worked better than I thought it would.
Could probably use it for a steam bath...
The experiment went ok and all but after the KMnO4 had dissipated (clear rxn mix) I suction filtered all the MnO2 off and kept the liquid which should
have been mostly K2CO3, potassium phthalate, and water. I added enough 35% H2SO4 to make the solution fairly acidic and got alot of bubbling so I
know the carbonate was decomposed and now I figure "ok, phthalic acid should precip much before the sulfate because of low solubility". So
I boil it down and nothing precips...damn back to the drawing board. Unfortunately this next semester will be a bitch and a half so I might be
waiting awhile before any MAJOR stuff happens other than passsive things like electrolysis....Overall a good holidays though!
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The traditional solution is to stop the cooling water in the condenser until the naphthalene melts and runs back into the mix. (Or poke it with a
stick).
|
|
|
Haggis
Hazard to Others
  
Posts: 238
Registered: 1-12-2002
Location: Mid-America.
Member Is Offline
Mood: Lacrymating
|
|
Distill Chloropicrin!?!
Wow. I hope you know what you can get yourself into, which you probably do, more than likely having dealt with the stuff before. Personally, I would
rather not get it more volitile as it already is.
I also assume you have a resipirator of some sort. Is it simple charcoal filtration? Good luck though, and post the results in the Chloropicrin
thread.
Great ideas often receive violent opposition from mediocre minds.
<b> <a href=\"http://pgp.mit.edu:11371/pks/lookup?op=get&search=0xEE41A2B1\">PGP Key</a> </b> 0C0A 7486 B97F
92EE AE50 A98C A4F3 087E 8CE9 A782
|
|
|
Al Koholic
Hazard to Self
 
Posts: 98
Registered: 2-12-2002
Member Is Offline
Mood: Seeking ligand
|
|
Traditionally that is true. I ran some steam through the coils of the condenser for a brief time and it seemed to melt the naphthalene much quicker.
Just turning off the water did let some fall back into the flask but also let more naphthalene get further up in the condenser...
The chloropicrin results will be posted don't worry! I have read a synth for guanidine starting from chloropicrin and ammonia/alcohol. Sure
would be a nice OTC way.
[Edited on 5-1-2004 by Al Koholic]
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
I've made Mg burn under water, but it took 6 bits with the last working. It flashed orange as it passed through the surface tension. More weird
color flashes: Way back in chem class when we burned some, my group's flashed green upon ignition and I saw one flash purple on a chem video
online.
<font size=4 color=purple><b>My 100<sup>th</sup> post!</b></font> 
|
|
|
Acid Test
fruitcake

Posts: 48
Registered: 6-1-2004
Location: Peterborough,Ontario,Canada
Member Is Offline
Mood: Flying High Aga
|
|
Silicone Oxide
Silicone Oxide is created from the fusion
of a silicone atom with oxygen.A great deal
of energy is required and no ameteur,novice
or general home lab would be able to produce
it.
A very high voltage is required(over 150,000)
and is created by normal sand clouds during
electical storms.
It can be created in a labratory with special
equiptment that is not accesable to any street
chemist.It is like trying to access a particle
accelerator or atom smasher.
.....
|
|
|
Mumbles
Hazard to Others
  
Posts: 436
Registered: 12-3-2003
Location: US
Member Is Offline
Mood: Procrastinating
|
|
And this has something to do with the topic how? This message goes to pretty much all your posts. Settle down, and quit posting garbage. Urinate in
a jar full of Bleach to generate chlorine gas? All I have to say is, WTF. Chloramine <i>maybe</i>. Maybe even Hydrazine if you're
lucky.
|
|
|
Acid Test
fruitcake

Posts: 48
Registered: 6-1-2004
Location: Peterborough,Ontario,Canada
Member Is Offline
Mood: Flying High Aga
|
|
My posts are far off from being garbage.
Likewise Hydrazine will not be produced
from NaOCl and urea.
Hydrazine is NH2-NH2 and is a three step
synthesis procedure from human urea to remove
the cabon and then remove the oxygen bond
between the two NH2 structures.Then of course
NH2 must bond with NH2.
I don't think this is possable from urea.
I would stick to getting hydrazine from
ammonia
.....
|
|
|
guaguanco
Hazard to Others
  
Posts: 216
Registered: 26-11-2003
Member Is Offline
Mood: heterocyclic
|
|
| Quote: | Originally posted by Acid Test
My posts are far off from being garbage.
Likewise Hydrazine will not be produced
from NaOCl and urea.
Hydrazine is NH2-NH2 and is a three step
synthesis procedure from human urea to remove
the cabon and then remove the oxygen bond
between the two NH2 structures.Then of course
NH2 must bond with NH2.
I don't think this is possable from urea.
I would stick to getting hydrazine from
ammonia |
Sorry, you're quite wrong:
hrdrazine synthesis<br>
hydrazine synthesis
[Edited on 7-1-2004 by guaguanco]
|
|
|
Acid Test
fruitcake

Posts: 48
Registered: 6-1-2004
Location: Peterborough,Ontario,Canada
Member Is Offline
Mood: Flying High Aga
|
|
As i have pointed out in the HCl and Cl2
subcategory of this forum(read it)
Hydrazine is produced in this manner:
2NH3+NaOCl+heat=NH2-NH2+NaOH+Cl
The addition of chlorine gas is unnessecary
for the synthesis of hydrazine.It is complete
in the procedure.Chlorine will dissolve into
the mixture while it is being heated.
This will crystallize as Hydrazine Chlorate
in the NaOH solution.
The creation of dissolvable Hydrazine Salts
with acids is unnesecary.Chlorine is an
acid and will create a dissolvable salt
.....
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
OMFG, can you research more please and quit speculating all of the time; it's fine sometimes though. Yes, I did the same when I first came here,
but then I made an effort to find really what mechanisms were driving things "behind the scenes". Admitting you're wrong is a very
positive trait, especially for a scientist. I remember doing all of the above wrong when I thought that Na reacted with H<sub>2</sub> in
the <i>Sodium!</i> thread in the described conditions. Boy, I was quite embarrassed, and very sorry for pissing off Organikum.
Before NaOH and Cl<sub>2</sub> produces chlorates, it first produces hypochlorites. Btw, chloramines will likely be produced (this is how
<i>I</i> understand it currently, please correct me if I'm wrong somewhere):
NaOCl + H<sub>2</sub>O
<sub><s><</s></sub><u> <s> ></s> </u> HOCl + NaOH
NaCl + H<sub>2</sub>O
<sub><s><</s></sub><u> <s> ></s> </u> HCl + NaOH
HOCl + HCl <sub><s><</s></sub><u> <s> </u>></s>
H<sub>2</sub>O + Cl<sub>2</sub>
Cl<sub>2</sub>
<sub><s><</s></sub><u> <s> ></s> </u> 2Cl*
NH<sub>3</sub> + Cl* <s> ></s> NH<sub>2</sub>* + HCl
NH<sub>2</sub>* + Cl* <s> ></s> NH<sub>2</sub>Cl
NaOH + HCl <s> ></s> NaCl + H<sub>2</sub>O
I think NH<sub>2</sub>Cl is less basic than ammonia because the electronegative chlorine atom pulls the lone pair closer to itself than
that found in ammonia. This would make it less accessible to a proton. Because OH<sup>-</sup> is still left over from some NaOH, and
amines are in equilibrium with water (NH<sub>2</sub>X + H<sub>2</sub>O
<sub><s><</s></sub><u> <s> ></s> </u>
NH<sub>3</sub>X<sup>+</sup> + OH<sup>-</sup> <tt>where X = Cl or H</tt> , the residual OH<sup>-</sup> forces the equilibrium more to the left which
favors products that can more easily go into the gas phase from aqueous. Hydrazine is also produced, but is very easily oxidized to less hydrogenated
products eventually having nitrogen gas leave the system. , the residual OH<sup>-</sup> forces the equilibrium more to the left which
favors products that can more easily go into the gas phase from aqueous. Hydrazine is also produced, but is very easily oxidized to less hydrogenated
products eventually having nitrogen gas leave the system.
|
|
|
Acid Test
fruitcake

Posts: 48
Registered: 6-1-2004
Location: Peterborough,Ontario,Canada
Member Is Offline
Mood: Flying High Aga
|
|
You are quite correct.
In fact NaCl+H2O requires a stronger
reaction procedure than normal heat.I think
that a current will do it.
You then draw off the HCl from the NaOH with
a glass baster
.....
|
|
|
Acid Test
fruitcake

Posts: 48
Registered: 6-1-2004
Location: Peterborough,Ontario,Canada
Member Is Offline
Mood: Flying High Aga
|
|
Thanks.. I was up all last night figuring out
certain hydrazine procedures and disproven
two.
I was referring to the equation NaCl+H2O as
a way of getting NaOH and HCl when there
is a need for it in the lab.
It requires the equiptment.I don't see a need
to add HCl to NaOH to make salt water.
It is pointless
To me it sounds like a job for God
.....
|
|
|
Acid Test
fruitcake

Posts: 48
Registered: 6-1-2004
Location: Peterborough,Ontario,Canada
Member Is Offline
Mood: Flying High Aga
|
|
Thanks but i was up all night working out
theory on synthesis of hydrazine,determining
authenticity.I went through 4 seperate
procedures plus my own which was:
NH2-CO-NH2 + NaClO = NH2-NH2 + CO + NaO + Cl
I don't see where chloramine is being produced
The brownish in the reacted solution is the CO
It has to be expelled.The NaO has to be removed
This requires precise reactions to extract
the NH2-NH2 before you crystalize it with an acid
It is a trick equation.It is obvious that
chloramine is not produced.If this were boiled
it would create HCON + NH3 + NaO which would
react with the Formamide.This is why the
CO has to be removed.THAT IS CRAP
.....
|
|
|
Acid Test
fruitcake

Posts: 48
Registered: 6-1-2004
Location: Peterborough,Ontario,Canada
Member Is Offline
Mood: Flying High Aga
|
|
CO is a colourless gas but when it is
dissolved in a liquid it is a brownish
tinge,especially in the presence of other
substances.
It is a known pollutant and it causes
asphykiation.
I would think my theory is correct
Chemistsss want you to think NaO doesn't
exist.
.....
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Acid Test, are you taking the piss out of everyone??
Do you test acid by placing a drop of it on the back of your tongue, just like you test acetaldehyde? The simplest and easiest test for sure  
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
From Hydrazine and it's derivatives Copyright 1985: | Quote: |
Two different reactions have been proposed fro the conversion of urea into hydrazine with the aid of sodium hypochlorite. The older publications
assume that N,N'-dichlorourea and para-urazine are formed as intermediate products.... "Most steps are currently believed to have
semicarbazide as the intermediate product".... An N-chlorourea anion H2N-CO-N-Cl is believe to be an active intermediate in the reacation.
CO(NH2)2 + 2NaOCl ----> CO(NHCl)2 + 2NaOH
CO(NHCl)2 + 2NH3 ----> RING(-NH-NH-CO-NNH2-CO-) +H2SO4 ----> N2H4*H2SO4 + 2CO2
|
The urea processes can be used with the Bayer process using ketazines for seperation.
|
|
|
Acid Test
fruitcake

Posts: 48
Registered: 6-1-2004
Location: Peterborough,Ontario,Canada
Member Is Offline
Mood: Flying High Aga
|
|
Chemleo-I test an acid by putting it on a piece
of cigarette foil.
I test for a base by diluting in 1ml of
water and dropping it on my tongue.It is
a surefire test.
KOH will tingle with a solid on contact.
I am not stupid.
Anything below KOH is probably strong.I wouldn't
Even attempt to test a solid OH below KOH without
diluting it.
Bromic Acid:
I did the Bayer-Whiff procedure last night
with a batch of my new procedure for
treating urine.I treat it with NaOH.Stand 24h.
Baste out liquid-NO PRECIPITATE-It contains
precursers for barbituric acid.The real
percipitate which forms 8 hrs later will smother
this just don't collect any precipitate.
(Optional-Treat with an alcohol of choice,besides pentyl alcohol)
Wash with Pentyl Alcohol or approriate solvent.
(Don'T use Naptha.Reserve this for clean
washing,not for removing waste out of urea.
Naptha can be reused for a period of time when
working with iso-lysergic acids from seeds)
Remove layers of waste with baster.
Add 2 oz of water and 1 oz of lemen juice
and shake
Liquid should be aqua clear
Put liquid in new flask.Add equal part NH3
Heat on med-low heat until mixture stops
giving ammonia smell.You should have 1/2
of what you started with.
Next cool down-and collect your thoughts and
perceptions because you'll need them.
Take 1 oz of this newly created urine and
simmer on med-low heat with a metal catalyst.
I used Al
When it is about to boil away stick your nose
over the pot.
A FAINT WHIFF OF AMMONIA
This is not excess from above procedures.
If you think this is so do another ounce.\
Smell at the begining of boiling...No NH3
I think this patent is a bogus
It is a patent for the manufacture of ammonia
for making hydrazine.
.....
|
|
|
| Pages:
1
2 |