aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
Grignard Degradation Mechanism?
So I'm attempting to figure out how the Grignard degradation works, I can't find it in any of my textbooks, and a brief recent literature search
doesn't turn up anything.
In a paper (see ref below), the authors took dimethylamino sulfonyl (DMAS) protected 4,5-diiodoimidazole, reacted with EtMgBr in DCM, and then
intentionally hydrolyzed to give only the 4-iodoimidazole.
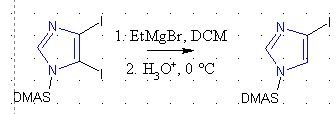
Now for a sanity check, the EtMgBr *should* react with the most electrophilic center (in fact shouldn't the Grignard react with the solvent?!). Why
would reacting and then quenching, cleave off the most reactive halogen, when the C-C bond is strong and can't simply be hydrolyzed away.
Even if by some leap of thought a Grignard is formed with the imidazole ring, would the magnesium insert itself between the C-I bond, or would the
entire MgBr replace the iodine in a metasthesis reaction?
What is more irritating is that the authors don't even address what the particular reaction is, so I'm a little worried I have it by the wrong name,
but what information I could find said that the Grignard degradation is used to sequentially remove halogens by reactivity.
Bhandari, M.R., Sivappa, R. et al. 2009. Total Synthesis of the Putative Structure of Nagelamide D. Organic Letters 11:7 1535-1538
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aonomus  | | So I'm attempting to figure out how the Grignard degradation works, I can't find it in any of my textbooks, and a brief recent literature search
doesn't turn up anything. |
Never heard about the phrase "Grignard degradation" used for this reaction. Try using the normal name for this reaction: magnesium-halogen exchange or
Grignard metathesis. It is a pretty common reaction. It is however uncommon to use it for hydrodehalogenations. More commonly it used to prepare
grignard reagents that are otherwise hard or impossible to prepare. Isopropylmagnesium chloride is most commonly employed and this way you can prepare
even "impossible" grignards like methoxycarbonylbenzenemagnesium halides, for example (the COOMe group generally slowly reacts with grignards at room
temperature, but the Mg-I exchange is rapid enough even at -20°C where such grignards last much longer). For example:
p-MeOCO-C6H5-I + iPrMgCl => p-MeOCO-C6H5-MgCl + iPrI (at -20°C)
This is essentially analogous to the lithium-halogen exchange reaction which however occurs much faster even at -78°C.
| Quote: | | Now for a sanity check, the EtMgBr *should* react with the most electrophilic center (in fact shouldn't the Grignard react with the solvent?!). Why
would reacting and then quenching, cleave off the most reactive halogen, when the C-C bond is strong and can't simply be hydrolyzed away.
|
The Mg exchanges the most exchangable iodine which is always the one which has the lower C-I bond energy and/or has neighbouring chelating groups (the
DMAS protection in this case). I do not know why they used CH2Cl2 as solvent, but there is no reason why not to use it. It does not react with
organomagnesium compounds at any useful rate - actually only very few alkyl chlorides react with grignards at all (without transition metal
catalysis).
| Quote: | | What is more irritating is that the authors don't even address what the particular reaction is, so I'm a little worried I have it by the wrong name,
but what information I could find said that the Grignard degradation is used to sequentially remove halogens by reactivity. |
Currently I do not have the time to check that reference, but I'm sure you will be able to find plenty of literature regarding this reaction if you
only use more proper keywords in your search.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
Thanks for pointing me down the right track, I'll look into it when I've gotten more sleep.
As for the solvent issue, I've always made Grignards in diethyl ether before, and 3am was probably another factor.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
They probably use DCM to solubilize the imidazole. I'm also willing to bet they used a commercially available grignard reagent solution, so the ethyl
grignard was probably not prepared in DCM. As for whether to draw it as MgBr or MgI, this is of little consequence. Not to mention that the
mechanism of metal halogen exchange is debated, it would have no effect on the outcome of the product.
The more important question to answer is: Why does it exchange that particular iodine, and not the other?
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
Selectivity is always due to either a steric effect, or electronic effect. I spoke with my professor last week and he was siding towards the DMAS
group having an electronic effect, but since the mechanism of the magnesium-halogen exchange is debated on and isn't known well, the
chelation/coordination proposal by Nicodem seems more reasonable.
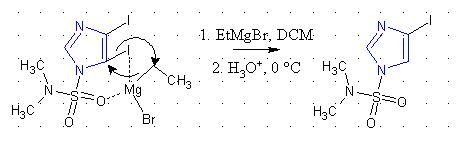
It seems reasonable that the electronegative oxygens on the sulfonyl may attract the magnesium, but the arrow pushing described is just proposed,
could be a radical mechanism, or just the wrong way around (I'm a little busy to look up whether a C-I bond is more electrophilic/nucleophilic than a
C-Mg bond).
|
|
|
|