| Pages:
1
2 |
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
piperazine from ammonia?
I believe this reagent was discussed previously on this website, if you use the search engine you may find it.
Reduction of pyrazine would be a good option, following the procedures for reducing pyridine to piperidine. Alcoholic ammonia with 1,2-dichloroethane
might be a bit messy. The action of di-sodium ethane-1,2-dioxide on ethylenediamine hydrochloride.
Pyrazine can be obtained from N-(hydroxyethyl)ethylenediamine by thermal dehydrative dehydrogenative cyclization. The precursor can be obtained
ethylenediamine and chloroethanol.
|
|
|
kerousel
Harmless

Posts: 23
Registered: 23-8-2009
Member Is Offline
Mood: No Mood
|
|
You know you're right search engine would show it, I just realized on wikipedia that this is already an established method of making piperazine...
Here's another question. I know that piperidine can be used to change a OCH3 methoxy group to an OH hydroxy group... would piperazine be able to do
this also? It would be nice since piperazine is a lot easier to make than piperidine.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kerousel  | | Here's another question. I know that piperidine can be used to change a OCH3 methoxy group to an OH hydroxy group... would piperazine be able to do
this also? |
What are you talking about? Provide a reference!
|
|
|
kerousel
Harmless

Posts: 23
Registered: 23-8-2009
Member Is Offline
Mood: No Mood
|
|
Well this is not what I need it for, but this is where I got the idea:
http://www.erowid.org/archive/rhodium/chemistry/codeine2morp...
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
There is no mention of piperidine there. There is described one example of aryl methyl ether cleavage using pyridine hydrochloride. Pyridine
hydrochloride is nearly not the same as piperidine. I recomend you to get more familiar with chemical nomenclature. Meanwhile, you can also use
ChemDraw to translate IUPAC chemical names into structures and vice versa.
|
|
|
kerousel
Harmless

Posts: 23
Registered: 23-8-2009
Member Is Offline
Mood: No Mood
|
|
Oh right pyridine. And this whole time I thought it was piperidine...
Wait a second then I don't understand how it works. The nitrogen probably takes the methyl group and breaks that double bond with the carbon... the
carbon now needs a new hydrogen atom... and the O still needs a new hydrogen atom- it doesn't add up!
edit: you know I don't even get how ether cleavage works then. what happens to the pyridine? Idk. I'll probably have to do some more research into
this. thanks.
[Edited on 25-8-2009 by kerousel]
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Perhaps, and I am speculating that pyrazine, which is basically pyridine with an extra N in the ring, i.e. an aromatic heterocyclic amine. Could
perhaps be used like pyridine if pyridine itself is unavailable. I posted a route to make pyrazine. I think it could be done "OTC" with a little
thought and patience.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
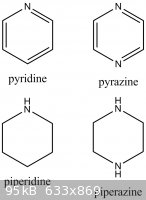
Ah. [Piperazine] it will probably do (pyrazine maybe if conditions are oxidizing or if some carbonyl is around to form Schiff bases)...in low yield
with a large amount of variously substituted amines (it can add multiple times). Yields will be bad and (I suppose) the mixtures intractable.
I attached the structures for clarification.
Cheers,
O3
[Edited on 25-8-2009 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
kerousel
Harmless

Posts: 23
Registered: 23-8-2009
Member Is Offline
Mood: No Mood
|
|
Oh alright, thanks. I would think that pyrazine should work for sure, then again I'm not sure exactly how pyridine works to cleave an ether at all.
But at that rate it might just be easier making pyridine from acetaldehyde and ammonia.
[Edited on 25-8-2009 by kerousel]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kerousel  | | Oh alright, thanks. I would think that pyrazine should work for sure, then again I'm not sure exactly how pyridine works to cleave an ether at all.
|
Pyrazine has the pKa of 1.1 so it is not like you can make a stable hydrochloride to be used instead of pyridine hydrochloride in methyl aryl ether
cleavage.
The mechanism of aryl methyl ether cleavage is SN2. You can find examples and further details about this mechanism in any organic chemistry book, or
Wikipedia in case you are too lazy to visit a library. How pyridine hydrochloride works in this cleavage, I explained already in detail in some other
thread, so please UTFSE.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Happyer
Harmless

Posts: 8
Registered: 23-12-2009
Member Is Offline
Mood: No Mood
|
|
Panziandi- You say 'Alcoholic ammonia with 1,2-dichloroethane might be a bit messy.' What do you mean by this?
Would refluxing 1,2-dichlororethane with alcoholic ammonia for a couple of hours be enough to produce piperazine or would you need pressure?
How about substituting the 1,2-dichloroethane for 1,2-dibromoethane as that is easier to make- pass ethene through bromine liquid.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Piperazine can be had cheaply at vet supplier for use as wormer.
|
|
|
Happyer
Harmless

Posts: 8
Registered: 23-12-2009
Member Is Offline
Mood: No Mood
|
|
Yes but this is for educational knowledge, i am not interested in obtaining large Qtys as it is listed here in the US.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Happyer  | | Yes but this is for educational knowledge, i am not interested in obtaining large Qtys as it is listed here in the US. |
What are you talking about? Piperazine listed? Listed as what, where and why?
I was told several times that in the USA piperazine salts are just as OTC as here in Europe or anywhere else for that matter.
|
|
|
Happyer
Harmless

Posts: 8
Registered: 23-12-2009
Member Is Offline
Mood: No Mood
|
|
I have been told the sale of large quantitys of freebase piperazine or salts are wached due to its possibility of being turned into illict substances.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
How could one turn piperazine or its salts (most likely hydrochloride) into "illicit substances"? Do you know of any such reactions and products? It
does not appear obvious.
PS: I was thinking of "harder" drugs than benzylpiperazine (BZP), which is still legal in many places and only recently criminalized here in New
Zealand; i.e. opioids, cocaine, amphetamines, and similar, especially those complying with the "morphine rule" for psychoactivity, i.e. an aromatic
ring, attached to a quaternary carbon, itself attached to a chain of 2 or more carbons, with this attached to a tertiary-amine nitrogen.
[Edited on 24-12-09 by JohnWW]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Presumably benzylpiperazine is the recreational drug in question (though there are plenty of other closely related compounds that are also
psychoactive). Of course this doesn't address the question of whether there is an easy synthetic route from piperazine to benzylpiperazine, but it
seems plausible enough (not going to research it right now).
(addendum, edit): The benzylpiperazine synthesis from piperazine is fairly trivial; you can find it in Organic Syntheses.
[Edited on 24-12-2009 by bbartlog]
|
|
|
Happyer
Harmless

Posts: 8
Registered: 23-12-2009
Member Is Offline
Mood: No Mood
|
|
JohnWW - Benzylpiperazine is the most obvious drug however there are lots of derivatives which are very trivial to make, hence i am being cautious.
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Well I said "messy" because it is unlikely that piperazine would be the sole product. In fact you are likely to end up with a majority of
1,2-diaminoethane. Other reactions products would be Bis(2-aminoethyl)amine, Tris(2-aminoethyl)amine, 2-Piperazinoethylamine and
1,4,7-Triazacyclononane etc.
I chose 1,2-dichloroethane because, I was speculating, that by controlling the rate of reaction you may favour the formation of the piperazine.
1,2-dibromoethane would react faster, which may favour the formation of some of the other products, mostly 1,2-diaminoethane.
[Edited on 26-12-2009 by panziandi]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Its quite easy to see that the first product formed from a mixture of 1,2-dihaloethane and ammonia will be 2-haloethylamine. How this reacts further
depends on the proportions of other reagents present.
It can react with a molecule of 1,2-dihaloethane to give bis(2-haloethyl)amine.
It can react with a molecule of ammonia to form 1,2-diaminoethane (ethylene diamine).
These two products can react further with other molecules in solution. Essentially what you get is a mess. The only product I think you can control
the reaction to produce is ethylene diamine, done so by adding the 1,2-dihaloethane dropwise to a massive excess of ammonia.
Bear in mind that each amine-halide reaction forms a molecule of HX, so when calculating the quantity of ammonia to use remember to multiply by two to
account for neutralisation of HX species formed.
|
|
|
Happyer
Harmless

Posts: 8
Registered: 23-12-2009
Member Is Offline
Mood: No Mood
|
|
How do i seperate the piperazine from the other mess?
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Fractionation! Either fractional distillation and/or crystalisation!
B.ps of some of the compounds i think will be encountered:
Piperazine - Melting point 106°C, Boiling point 146°C
1,2-diaminoethane - Melting point 9 °C, Boiling point 116 °C
Bis(2-aminoethyl)amine - Melting point -35 °C, Boiling point 199-209 °C
Tris(2-aminoethyl)amine - Melting point −16 °C, Boiling point 265 °C
1,4,7-Triazacyclononane - Melting point 42-45 °C, Boiling point 110-130 °C/7 mmHg
2-Piperazinoethylamine - Boiling point 218-222 °C
Of course, if you did this on a large scale, you would obtain an acceptable quantity of the desired compound, plus quite a few other useful compounds
as by-products.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
The phosphate salt crystallizes nicely.
|
|
|
Happyer
Harmless

Posts: 8
Registered: 23-12-2009
Member Is Offline
Mood: No Mood
|
|
Ok, i have managed to source a product containing 4g of piperazine phosphate, Senna and colours + flavours.
How can i isolate pure piperazine from this? i was thinking product + caustic solution to freebase the piperazine followed by ether extraction, is
this right?
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Come on! You're not expected to go to the next library, but you could at least have read the wikipedia entry on piperazine: http://en.wikipedia.org/wiki/Piperazine
| Quote: | | Piperazine is freely soluble in water and ethylene glycol, but insoluble in diethyl ether. |
|
|
|
| Pages:
1
2 |