darel
Harmless

Posts: 27
Registered: 12-7-2008
Member Is Offline
Mood: No Mood
|
|
Pentaerythritol from erythritol
I am looking for a route to Pentaerythritol besides the acetaldehyde and formaldehyde combination. Would it be possible to synthesize Pentaerythritol
from Erythritol?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
The short answer is no!
Erythritol contains 4 carbons, one fewer than pentaerythritol. . .
But the difference in performance between ETN and PETN is slight and erythritol can be nitrated by KNO3/H2SO4 mixtures, whereas PETN preparation
requires strong, white HNO3.
[Edited on 16-6-2009 by hissingnoise]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Well, no, and why would you think so> Because of the names?
And why would you want to if you could? Because it would be uneconomical if it could be done, but it cannot.
Pentaerythritol
C(CH2OH)4
Erythritol, ignoring the stereochemistry:
HO-CH2-CH(OH)-CH(OH)-CH2-OH
Sic gorgeamus a los subjectatus nunc.
|
|
|
darel
Harmless

Posts: 27
Registered: 12-7-2008
Member Is Offline
Mood: No Mood
|
|
I'm not asking to turn straight erythritol into Pentaerythritol. Just wondering if it can be done using erythritol as one of the reactants. I have a
readily available supply of the stuff. It would be cheaper for me than the aldehyde route.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Look darel, if you had a ready supply of glycerin would you look for its conversion to ethylene glycol?
ETN is an extremely powerful explosive. . .
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
It's the wrong carbon skeleton.
You really seem hung up on names and pay no attention to structure.
Get a different hobby.
Sic gorgeamus a los subjectatus nunc.
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Quote: Originally posted by hissingnoise  | The short answer is no!
Erythritol contains 4 carbons, one fewer than pentaerythritol. . .
But the difference in performance between ETN and PETN is slight and erythritol can be nitrated by KNO3/H2SO4 mixtures, whereas PETN preparation
requires strong, white HNO3.
|
Petn does not require high concentrations of HNO3 , only the yields suffer. The only thing that really maters is theamount of water in the mix which
obviously can be altered.
What a fine day for chemistry this is.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Theoretically, almost anything can be turned into almost anything else on an industrial scale via partial combustion and carbon monoxide plus hydrogen
to methanol and so on.
You can also do it biologically... convert the carbohydrate to biomass and use that as a feedstock.
But it isn't anything you can do with test-tubes and flasks in the chem lab.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
This is a stupid exercise in futility. On any scale it is hard to get cheaper than formalin and acetaldehyde. Instead the thread author wants to take
a really unrelated (structurally) carbohydrate snd make pentaerythritol out of it, He might as well start with methane.
What is worse he is too lame to find out for himself that this is a brainless idea, and is inviting the forum to jump through hoops at his behest in
quest of his magic process.
No thanks, fella. Try learning some chemistry so you won't waste our time like this.
[Edited on 17-6-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
You could not use erythritol as a reactant.
Look:

That's PE

That's erythritol
Pentaerythritol has no stereochemistry, and is far diffferent from erythritol. Erythritol is a sugar alcohol: It is derived from trees. The only
similarities are that they are both polyols. Doing anything like this biologically is foolish. It either takes enormous amounts of time, or it costs
thousands of dollars.
The Aldehyde process looks fairly simple, as all the precursors are quite easy to obtain. Calcium hydroxide, formaldehyde, and acetaldehyde. If you're
that desperate, I believe Sigma sells 1 KG of PE for around $30.
[Edited on 17-6-2009 by Rich_Insane]
[Edited on 17-6-2009 by Rich_Insane]
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
If you find that you can not get acetaldehyde and formaldehyde or buy the one kilogram of PE, the aldehydes can be made with the partial oxidation of
the corresponding alcohols, which are ethanol, and methanol.
|
|
|
The_Davster
|
Thread Moved
17-6-2009 at 13:49 |
Siconic
Harmless

Posts: 10
Registered: 27-3-2009
Member Is Offline
Mood: No Mood
|
|
Convert Erythritol to Pentaerythritol?
Is it possible to convert erythritol to Pentaerythritol? Just a question, although it seems stupid since Pentaerythritol is obtained from condensation
of acetaldehyde and formaldehyde. Just figured if there is a way it would be cheaper since erythritol is dirt cheap.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Even if you could, which you can't, why would you want to?
There's very little to choose between ETN and PETN, performance-wise.
|
|
|
Bolt
Hazard to Others
  
Posts: 188
Registered: 26-3-2007
Member Is Offline
Mood: No Mood
|
|
That's not true: ETN is much, much more sensitive to shock than PETN. Also, eutectic mixtures will have different properties
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
I said nothing about properties. . .
|
|
|
per.y.ohlin
Harmless

Posts: 27
Registered: 14-11-2009
Member Is Offline
Mood: No Mood
|
|
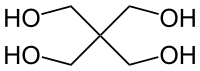
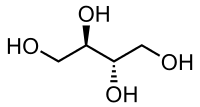
They have a different carbon backbone. Just because they have a similar name does not mean they are chemically related.
EDIT: There is already a thread on this: http://www.sciencemadness.org/talk/viewthread.php?tid=12380
[Edited on 21-3-2010 by per.y.ohlin]
[Edited on 22-3-2010 by per.y.ohlin]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Yeah, I did think I was repeating myself!
|
|
|
Nicodem
|
Threads Merged
21-3-2010 at 12:14 |