green
Harmless

Posts: 15
Registered: 30-3-2009
Location: South Dakota, USA
Member Is Offline
Mood: No Mood
|
|
make a photocatalyst to respond to full light spectrum
titanium dioxide nanoparticles or nanotubes are used as photocatalyst in solar cell. There is a problem with that. TiO2 based catalsyt only absorbs
UV lights (5 % of the sun energy). I want my cell to respond to full light spectrum. One method is doping TiO2 with notrogen and other inorganic
molecules. I want to hear suggestions.
[Edited on 11-4-2009 by green]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
You are aware that most TiO2 photovoltaic system us a dye sensitiser? The absorption spectrum is mainly determined by the dye and its coupling with
the TiO2
See attached image for an example, a 7% efficient cell using a tailored zinc phorphorin for the dye.
If you really want to nitrogen dope TiO2, heating it to 900 C or so in low pressure NH3 might do it, although you'll likely need an extended bake
under argon to allow the nitrogen to diffuse from the surface.
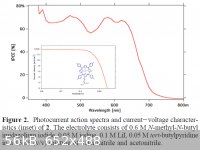
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Apparently soaking the TiO2 coated (its only a thin film right?) in raspberry juice does the trick. At least thats what I remember from an open day
visit tp Sussex university here in the UK.
|
|
|
green
Harmless

Posts: 15
Registered: 30-3-2009
Location: South Dakota, USA
Member Is Offline
Mood: No Mood
|
|
what is the source for the attached image?
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
I have both anatase grade pigment, and a Hombikat nano powder, but my application is PCO (in house & in vehicle photo catalytic oxidation). This
is does quite nicely under UVA black light (mineralises small organic dust particles and VOC's quite efficiently). Not sure, but a doped TiO2 (which
responds to fuller spectrum) might have to be sintered, and initially formed using a nasty, TiCL4. There is a start up company, now manufacturing
these ultra thin cells (very high area to potential conversion), and the TiO2 cells appear red. These will compete with the CIGS undoubtedly.
Remember, I think the pearl'escent 3M ribbon stuff (which looks sort of like mother of pearl) is based upon TiO2 chemistry.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Maybe this will help. I just came across it when trying to back up the catalyst archives. Anyone want to help me? Its full of good information and we
only got 9 days left to archive 126 volumes.
TiO2 Nanoparticles with High Photocatalytic Activity Under Visible Light
Attachment: fulltext6.pdf (394kB)
This file has been downloaded 675 times
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
J. Phys. Chem. C, Vol. 111, No. 32, 2007
DOI: 10.1021/jp0750598
Highly Efficient Porphyrin Sensitizers for Dye-Sensitized Solar Cells
Wayne M. Campbell,† Kenneth W. Jolley,† Pawel Wagner,† Klaudia Wagner,† Penny J. Walsh,‡
Keith C. Gordon,‡ Lukas Schmidt-Mende,§ Mohammad K. Nazeeruddin,§ Qing Wang,§
Michael Gra1tzel,§ and David L. Officer*,†,£
attached paper, supporting data is free access on-line and too big to attach.
[Edited on 12-4-2009 by not_important]
Attachment: n1.pdf (62kB)
This file has been downloaded 1540 times
|
|
|
jokull
National Hazard
   
Posts: 506
Registered: 22-2-2006
Location: Everywhere
Member Is Offline
Mood: Ice glassed
|
|
In a solar cell TiO2 does not work as a photocatalyst but as a charge generator and carrier.
In order to photoexcite a material, in this case a metal oxide, it is neccessary to irradiate it with light whose energy is equal or greater than its
band gap. Band gap is related to several factors such as particle size and crystal perfection.
For example, bulk ZnO and TiO2 are UV-responsive materials but if you get nanoparticles then their band gaps are reduced by a few electronvolts, i.e.
they can be excited with visible light.
In photocatalysis, recent efforts are conducted to use visible light as excitation source, so that sunlight can be "harvested". However, the big deal
is not "how easily a photomaterial can be excited" but "HOW EFFICIENTLY THE GENERATED CHARGES ARE USED".
A "full spectrum" responsive material would be GREAT, however there are physical concerns that you have to know and consider for your designs.
|
|
|
argyrium
Hazard to Others
  
Posts: 123
Registered: 3-2-2008
Location: Pacific
Member Is Offline
Mood: No Mood
|
|
Slightly afield, but some related information follows.
For some time, I have been interested in anthocyanin dyes; specifically ternatins/dephinidins found in the inflorescence of _Clitoria_ternatea_
(Leguminosae).
The color of the fresh flowers could be described as a "hurt-your-eyes- BLUE". Very intense, with a peculiar color shift (to purple/red) dependent of
color Temp. of illuminating light more dramatic than any other I have ever seen (I work with powdered pigments and dyes on a daily basis). Simple
extracts made by me also exhibit considerable light stability. Color of the applied extract is similar to natural indigo.
Most natural blues either fade rapidly or turn brown/grey and/or both. The blue from _C._ternatea_ remains quite vivid in EtOH and H2O solutions as
well as dried on filter paper exposed to direct sunlight (Hawaii) for ~8 hrs/day.
It apparently is used in some sort of a beverage in Thailand (input Sauron???).
It has been investigated as a food colorant, antioxidant, etc, as well as the possible use as in dye-sensitized solar cells over a TiO2 substrate.
q.v.
Attachment: Ts-3 dye sensitized solar cell using natural dyes extracted from rosella and blue pea flowers.pdf (162kB)
This file has been downloaded 907 times
I have another file related to this somewhere and will post when located, also some images.
Argyrium
|
|
|
argyrium
Hazard to Others
  
Posts: 123
Registered: 3-2-2008
Location: Pacific
Member Is Offline
Mood: No Mood
|
|
Color in daylight*; side-lit.

Color under tungsten light*, side-lit.

Other DSSC paper:
Attachment: solar cell1JPhysChemB.pdf (496kB)
This file has been downloaded 570 times
Argyrium
*Images were adjusted to closely match actually perceived color shift which can often be even more dramatic than what is shown.
[Edited on 12-4-2009 by argyrium]
|
|
|
green
Harmless

Posts: 15
Registered: 30-3-2009
Location: South Dakota, USA
Member Is Offline
Mood: No Mood
|
|
thank you for the great discussions and suggestions.
hey Argyrium those are really cool images you have posted.
|
|
|
argyrium
Hazard to Others
  
Posts: 123
Registered: 3-2-2008
Location: Pacific
Member Is Offline
Mood: No Mood
|
|
They are very attractive flowers but only last over-night when put into water.

Globey, what is the "pearl'escent 3M ribbon stuff" you refer to? Are you speaking about one of the interference pigments or some PV
specific material?
Thanks
Argyrium
|
|
|
Texium
|
Thread Moved
19-11-2023 at 12:58 |