dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Measuring pH in a Chlorate cell
Hello,
My pH meter has given up the Ghost. (failed to function), so I will have to splash out and get another one.
Can anyone suggest an indicator that would be able to function in a sample of cell electrolyte taken from the cell.
Wiki page on indicators here.
http://en.wikipedia.org/wiki/PH_indicators
A home made indicator would be fantastic (boiled caggage sounds good!) though I do not think that is possible as it will be destroyed by the
Hypochlorites etc
Strip indicator (as pictured on the Wiki page at right hand side) works OK so long as you read them quickly but they are expensive in the long term.
You would be better off buying a pH meter.
The cheaper 'narrow strip of paper in a roll soaked in indicator' do not work at all. At least the few ranges I have do not.
I was also thinking of perhaps holding wet pH paper in a stream of the fumes coming from the cell. The amount of Cl gas in the vent gases may be a
usable indicator of cell pH.
Perhaps measuring the O2 coming from the cell would do the thrick but I don't think that would be too simple/cheap.
Dann2
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
A cheap soil pH meter might work---but you'd have to clean it thoroughly after every dipping.
Worth trying, though?
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Thanks for that. I will purchase a ph meter as I want to have one anyways. I always washed the one I had after each measurement. I have it ten years
so I suppose it was getting old. It was a professional, 'carry in your top pocket' type.
I tried BromoCresol Purple and it held a purple colour for a few seconds in a cell sample but since I have no ph meter I am kind of guessing the pH.
The pH strips that I have are not very good at showing up small variations in pH values.
Perhaps it would be possible to slow/stop pH rise (as you suggested in other posts) by trying to react the escaped Cl with the Cathode Hydrogen but
thats for later I guess.
Would you need 'hard' or 'soft' UV to hurry up or get the reaction to happen.
Dann2
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Perhaps there is some way to monitor the cell conditions with voltammetry? Perhaps using a platinum or iridium wire sensing electrode....you just need
some reproduceable setpoint to control automatic addition of HCl?
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
Thanks for replies. I think it may be a bit of a pipe dream trying to monitor/meaure pH by using something other than a pH meter!
What is Voltammetry?
Do you put two inert (Pt?) electrodes into the solution and then scan the electrodes with a Voltage that goes from (say) -4 to +4 Volts and plot the
Amperage that you get on one axis and the Voltage on the other axis? The -4 to +4 may be too much as amperage may be very large in that case use a
narrower Voltage range.
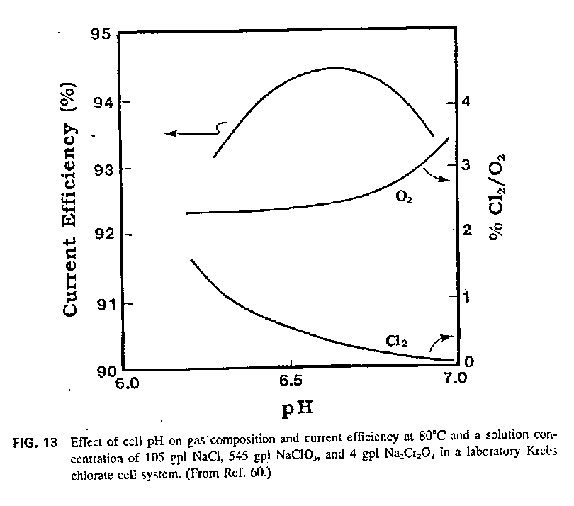
Since the graph above seems to suggest that Oxygen leaving the cell is dependend on pH (like the Chlorine) then I wonder would an Oxygen sensor from a
car (cheap from car parts supplier or cheaper from scrap yard) measure the Oxygen content of the exhaust gases and give an indication of pH. I can see
the car sensor getting poisoned fairly quick. You could put the sensor at the top end of a condensor tube (a tube jacketed with water transporting
exhaust gasses away from cell) so that no dreaded Chlorate+Chloride containing cell mist would get near the sensor. If Chlorine gas does not poison
these sensors then it may work.
Dann2
What do you call a boomerang that will not come back.
>>>>>>> A stick.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Dann2, I thought we had given up the notion of continuous immersion of a pH probe as basically untenable for a home cell, as the cost of pH probe per
kg chlorate produced would be astronomical. I'd still like to find out how the commercial plants do it. My gut feel is that they use cheap probes
and essentially throw them away after a single run that would produce perhaps tons of sodium chlorate, lowering the cost overall of the probe's loss.
I've been using a double junction probe, drawing off samples, and checking the pH as if the sample was liquid pH-probe death; instant, vigorous
washing after the measurement. So far, it's hung in there.
How about this - complex but possible. Assume (or determine) the CE of a particular cell. Then, create or program a microprocessor-based device that
adds acid based upon the current delivered. The additions can be spaced, or continuous but very slow, but essentially tie in the known requirement of
HCl per amp, and work from there. Back up the device with occasional checks of pH. I guess the problem there is that pH swings, and the need for
HCl, vary over the cycle. Towards the end of the cycle, less acid is needed. So scratch that concept.
Extrapolating from ^^^. With a given cell, manually track the HCl additions to keep the pH in the proper range. Next run, the programmable unit
replicates your hand delivery of the HCl. With all else being equal, it could automate a tedious task.
I do remember a patent somewhere that discusses the use of iridium or some other relatively exotic microprobe that measures the potential across a set
of electrodes, and from there, pH and acid need can be determined. I'll see if I can dig it up.
The O2 sensor is an interesting thought.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
My pH meter stopped functioning some time ago. It was about 10 years old so I cannot blame the Chlorate cells.
I have purchased a cheap one same as this.
How long it will last we will see. I wash immediately after using too.
I think the Chlorine or Oxygen monitoring won't work as the amount coming out of the cell will change in sympathy with the particular stage of the
cells progress (concentration of Chloride/Chlorate. This would be difficult to relate to pH of the cell unless a heap of data was gathered first as
you suggested.
Are there any pH strips out there that are more resistant to the bleaching effect of the cell contents. It's a bit of guesswork trying to read the pH
strips that I have, they are good quality 'four colour' one's.
Dann2
[Edited on 10-4-2009 by dann2]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
sorry to necro this but I am currently actually working on a 100A chlorate cell and the way pH measurements will be done is that firstly its a Ca ion
at 8g/l buffered cell which already keeps the pH at 6.5 but HCl will still be needed to be dripped in at a constant rate to replenish the buffer. When
you run a cell at correct pH there is a noticeable voltage drop if constant current is applied on electrodes assuming connections arent faulty. This
new voltage then seems to run at steady state til the chloride levels drop much later in the run. So the means of checking the pH will be by the use
of 2 carbon small electrodes that will run at constant voltage at 1.5V and the current will vary if incase the buffer is exhausted or too much HCl is
being used ideally. The probe will also tell if the run is approaching low chloride levels as the buffering effect of Ca diminishes and pH change will
occur thus drop in current will be seen on the probes.
The use of this chlorate is purely for bleaching purposes so dont get your hopes up for any videos of 2kg chlorate mixtures or something silly. plus
it aint even hapenning in my soil.
[Edited on 23-4-2024 by mysteriusbhoice]
|
|
|