len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Calculations on the potency of poisons
Due to some work on CN got interested in the seat of extreme toxicity of certain substances. In the course of this I did some back of the envelope
calculations which I haven’t seen elsewhere - hence reproduce here.
Cyanide kills mammals (LD50) in a ratio of about 1mg/kg body weight, or about 80mg to kill an average human. How is cyanide able to affect in such
drastic fashion an amount of material one million times its mass? What is the seat of this amplification? CN- binds to the cytochrome c oxidase
enzyme which catalyses chemical energy generation via ATP in the mitochondria (semi-autonomous genetic and functional units) of cells. This enzyme
stores energy via the Fe2+->Fe3+ + e equilibrium, however in-vivo the iron is not free but bound to an extremely large protein of mass 10^5 au.
The CN- complexes the metal centre so it is no longer able to change oxidation states. Therein the seat of the amplification. 0.1gms of cyanide
corresponds to
10^5 (mass cytochrome) / 66 (mass KCN) * 0.1gms = 100gms of cytochrome destroyed.
Mitochondria typically take up no more than 10% of cell volume (1-10um diameter) and with enzymes being present typically at the level of 0.1-1%,
means the max total weight of mitochondria in a 100kg man (10kg) should contain 10-100gms of the enzyme – which correlates well with the toxicity of
cyanide. Our cells have evolved to acquire most of their energy from mitochondria, with the latter put out of action, only the lower efficiency
glycolysis energy generation mechanism remains – and energy hungry cells, such as neurons die.
Alarmingly there are far more potent poisons than cyanide active at a rate of 0.02-10ug/kg body weight corresponding 100 to 50000 times the potency of
cyanide. To these belong toxins such as curare, most snake venom, organophosphates such as sarin. All these substances work by paralysing skeletal
muscle control in the peripheral nervous system – their detraction is that they disable only a limited number of specialized cells in the chemical
synapses of the muscle – neuron cell interface, and if intensive care is long enough these are regenerated and the patient lives.
What is the reason for the amplification here?
The cytoplasm of a single neuron cell (which can be up to a meter long) conducts electricity (almost at the speed of light). However different
neurons are not connected to each other electrically, but rather chemically – with the signal passed from the axon of one neuron to the next through
the release of a chemical transmitter which permeates a 20-40nm gap between them, called the chemical synapse. The timescale of this is still very
fast for a chemical process due to the smallness of the gap, in the order of milliseconds. Due to its incredible smallness in relation to its
importance, a very small amount of chemical able to concentrate by binding at the synapse and interfering with its function is able to have an
out-of-proportion effect on the organism as a whole.
Different neurotransmitters are used at different types of neuronal junction and a given interferer or toxin can generally affect only a particular
type of synapse. For instance serotonin and dopamine are the chemical messengers at chemical synapses in the brain and a small amount of substance
such as morphine, cocaine, and LSD by binding and interfering at these synapses are able to exert a profound effect on the functioning of the brain.
The latter holds the record with profound effects seen at 0.5ug/kg.
All neuron to muscle junctions use an acetylcholine mediated chemical synapse, which are not bound to, and therefore affected by, the chemicals above
– a person who is ‘high’ still has low-level control of his muscles. They are however affected by substances such as sarin and botulinum, with
sarin inducing paralysis by disabling an enzyme responsible for moping up the acetylcholine – leaving the signal path always on, and botulinium
disabling the permeation of acetylcholine through the membrane at the synapse. Botulinum holds the record of 0.02ug/kg LD50 for injection in
mammals. An injection of an invisible 2ug speck of the botulinum protein will kill a human. Botulinum does not affect serotonin/dopamine synapse,
and the thought processes of a person so paralysed remain clear.
How do extreme potencies such as 0.02ug/kg result?
There are approximately 639 skeletal muscles in the human body, with on average several thousand muscle fibers (cells) per muscle. This makes about 1
million skeletal muscle cells, each connected to a single neuron – hence this is also the approximate number of acetylcholine-mediated synapses of
the PNS. The diameter of one such synapse is 1-1.5um, while its length is 20-40nm, assuming a cylindrical form we get 10^-19m3 for the volume of a
synapse, or 10^-13m^3 for their volume in the entire human body. A 100kg human occupies a volume of about 100L, i.e. 10^-1m3, and so the material in
synapses form 10^-12 of body weight. Hence the typical weight of an organic substance attached perfectly and completely filling all the synapses is
10^-7gms = 0.1ug for 100kg body weight. The actual figure of 0.9-1.5ug for botulinum is 10 times greater of course due to imperfect diffusion,
filling/attachment, decomposition etc, however the degree of potency is perfectly explained.
However there are more potent poisons still.
Polonium-210, made famous in a recent poisoning en.wikipedia.org/wiki/Litvinenko, has an LD50 of 0.7ng/kg by injection [current wikipedia is misleading in this figure, as is to be expected of a
constantly editable source] – which is 30 times more poisonous than botulinum, and 1.4 million times more poisonous than cyanide! Moreover it
destroys all body cells rather than disabling a small set, and its effects are irreversible. 50ng are sufficient to kill an average human - this
amount of matter is invisible to the naked eye.
Polonium releases a total 3*10^9 J/gm in the form of radioactivity.
J/gram = a-energy * eQ * Av / Mw
= 5 *10^6 * 1.6 * 10^-19 * 6 * 10^23 /210
=2.5 * 10^4 * 10^5 = 2.5 * 10^9, that is approximately 3 billion joules/gm.
Converting this to Sieverts for a 100kg man we have
20 * 2.5 * 10^7 Sv/g = 5 * 10^8 Sv/g
A dose of 4.5Sv is generally considered to be LD50
Hence for polonium an LD50 is 4.5/5 * 10^-8g = 10 ng
However, the amount of energy released by 50ng (150J) of Po-210 can only heat the body by 150/(4000*80kg) = 1/2000 of a degree! Yet it produces
complete shutdown of all vital systems in a matter of weeks with a failure of organs whose total mass exceeds that of the poison by 10kg/0.05ug = 200
billion. What massive amplifying effect, making the human body an exceptionally sensitive radiation detector is at work here?
It turns out that the mammalian cell is so sensitive to radiation that 200rad - an amount of energy sufficient to raise its temperature by only
0.0005C if delivered thermally - if delivered in the form of a hard charged particle is sufficient to kill it. Since nuclear processes are small
scale, they are quantum processes, and most process energy is typically ranged fairly narrowly, with emitted particles having energies in the range of
several MeV - about several 10^-13J per particle. But
this means a single radioactive particle (several nucleides maximum) can kill a cell consisting of 10^-9/10^-24 particles. Therein the massive
amplification factor of 10^15!
To irradiate all cells in a 100kg human body the amount of Po-210 needed is roughly
100kg * 10^-15 (mass particle/mass cell) * 210 (mass Po-210/mass particle) = 20ng
In excellent agreement with observed results!
What exactly happens on the chemical level is not fully understood - but the basics are known to come about from the fact that cells contain a suicide
mechanism (apoptosis) whose seat lies at the atomic level. A single atom incorrectly placed can lead to chemical reactions which on completion (this
‘amplification’ or ‘developing’ takes several weeks) in the cell lead to apoptosis. It also explains the existence of a 'walking ghost' phase
of several weeks where en.wikipedia.org/wiki/Walking_ghost_phase people who are definitely going to die from radiation go thru a period when they are apparently well -
their cells are still functioning - even though they have been irreversibly programmed to die. Death is inevitable as there is no known method of
reversing the apoptosis process once it has been started. [[en.wikipedia.org/wiki/Louis_Slotin]]
This amplification is not evenly spread among cells, Many bacteria cells can sustain an enormous amount of radiation before dying, to the extent
that they fry thermally first (completely the opposite of the case for human cells) and this explains why radiation can not be used as an effective
disinfectant - we are far more sensitive to it than anything else - radiation is good for homicide - it can rid wheat of people without spoiling it -
but not for insecticide or bactereocide (antiseptic). Even within the humans body not all cells are equally susceptible - cells stop replicating at
levels of radiation www.iaea.org/Publications/Magazines/Bulletin/Bull023/02305892124.pdf well before apoptosis is triggered - probably due to the huge DNA molecule
essential to replication, being sensitive to a single atomic change. This explains why radiation sufferers en.wikipedia.org/wiki/Litvinenko display loss of hair and immuno deficiency (death of fast dividing cells) before system shutdown symptoms appear
(massive cell apoptosis).
From the toxicological viewpoint there is nothing special to Po-210. Poisoning by radioactive materials is essentially all of the same type. What is
important is how long the average Po atom takes to decay with respect to how long on average it takes to be eliminated from the body, and its
detectability. In physical terms the important characteristics are the half-life (138days), type of radiation whether: electromagnetic-gamma,
electron-beta, neutron, helium nulceus (only alpha), and radiation energy range hard or soft (hard). Radium for instance, is on half life alone
1680yrs/138days ~ 4400 less poisnous than Polonium-210. Uranium is more toxic chemically than radioactively, being 4*10^9yrs/138days = 10 billion
less toxic than Po-210. On the other hand a very short half life, seconds or days typical for many isotopes, is detrimental to toxicity since the
toxin would be essentially non-radioactive before having a chance to be administered. Still there are hundreds of isotopes with a half-life in a
suitable range, Even more isotopes emit the most toxic hard alpha particle radiation (in converting the dose in J/kg to the Sievert, the empirical
biological effectiveness of radiation, alpha particles have the highest multiplication factor of 20).
Po-210 is perhaps most unusual in its detectability characteristics. Unusually there is no gamma or beta radiation (former occurs in one in 10^5
decays), while alpha being charged has a mean free path measured in microns in fluids and solids. So Geiger counters will not detect it. Its
radiation will not even pass through paper (100um thick). It can be taken through airport detectors. An invisible amount
[en.wikipedia.org/wiki/Lugovoi] 15ug of Po-210 will kill everyone on a plane, yet can not be detected on embarkation. Resonance spectroscopy which
can detect nitrates for instance, is not sensitive enough to detect such small amounts of matter, which can be inconspicuously EM shielded, in say a
pin head.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
One aspect of radioactives is that while the radiation emitted is in the range of 10s of KeV to 10 MeV, chemical bond energy is less than 10 eV. This
implies that a single decay could disrupt 10^3 to 10^6 bonds, a significant amplification factor.
With alphas in a certain energy range, for faster ones after they've been slowed down a bit, this bond breaking is going to happen in a very small
region. If this is near a DNA strand multiple points of damage to the DNA can be done in a short stretch, damage exceeding the repair mechanism
ability to fix. It may be that significant damage could be done to cell organelles, again past the point that the cell can repair before the damage
interfers with cell functioning.
In metazoa it makes sense for cells with cells with such damage to shut down. Normally damage like that would be the result of a cosmic ray or
spontaneous fission of a actinide that happened to be in a cell or very near it; these are rare to extremely rare event. Metazoe have lots of cells,
in most the lost of one is insignificant, so damaged cells can be programmed to self destruct or be destroyed by other cells. Bacteria don't have
that luxury, even abnormal cell multiplication is better than no cell; while such abnormal growth in a metazoe may be disruptive - cancer.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
| Quote: | Originally posted by not_important
One aspect of radioactives is that while the radiation emitted is in the range of 10s of KeV to 10 MeV, chemical bond energy is less than 10 eV. This
implies that a single decay could disrupt 10^3 to 10^6 bonds, a significant amplification factor. |
If all the energy of a charged particle of say 10MeV went purely into breaking electro(chemical) bonds in a block of material it was fired at, it
would break of the order of 10^6 bonds and the material remain as cold as it were before. In fact the charged particle does not encounter target
atoms one by one, but rather the shock spreads as a wave in a 3D lattice so materials bombarded with radiation heat up manifesting more than 99% of
the injected energy as heat energy - thats why the thermal comparison is interesting - this energy will heat the cell up only 0.0005 degree. The
remaining energy goes to breaking 10^4 or so chemical bonds, but as I wrote the cell contains about 10^15 atoms so a factor of the order of 10^11 must
still be explained by apoptosis. That this is so is extremely evident - very little fractional damage is done to the cell by a single energetic
particle - one can not find any change in organic mater such as plant and bacteria on being bombarded by single energetic particle per cell. Humans
however die. Radioactive decay energies are widely ranged overall, but most nuclear decays, especially alpha decays, are clumped in the MeV range.
| Quote: |
In metazoa it makes sense for cells with cells with such damage to shut down. Normally damage like that would be the result of a cosmic ray or
spontaneous fission of a actinide that happened to be in a cell or very near it; these are rare to extremely rare event. Metazoe have lots of cells,
in most the lost of one is insignificant, so damaged cells can be programmed to self destruct or be destroyed by other cells. Bacteria don't have
that luxury, even abnormal cell multiplication is better than no cell; while such abnormal growth in a metazoe may be disruptive - cancer.
|
I think thats because short lived few cell organisms have much less chance of cancer developing before they die - with mutations assumed a linear
function of time. A single-cell bacteria living for 1 day on this basis has 1/(36500 * number cells in human body) the latter being of order 10^14,
less chance of developing cancer than a human organism. This explains the extreme sensitivity our cells have developed to cell damage - and still its
not enough as 1/3 of us succumb to cancer. In radiation poisoning unfortunately this extreme sensitivity makes us equally extremely vulnarable
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Here's an extract I found in a medical book wjich supports my point above regarding the small fraction of a high energy particles energy which
actually goes to bring about chemical changes
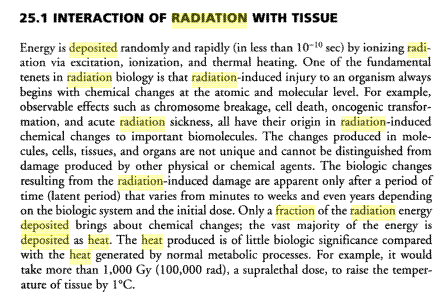
|
|
|
|