Isomeric_Fred
Harmless

Posts: 31
Registered: 25-6-2006
Member Is Offline
Mood: curious
|
|
Cinnamamide purification
Hello. Long time no post, but I find myself in need of help and this is the natural place for me to ask questions.
So I m trying to find a suitable purification method for cleaning cinnamamide from a mixture of dimethylacetamide and acrylamide and some
bromobenzene.
The m.p. of cinnamamide is around 140ish degC so distilling is out of the questions. I dont feel like running a flash column but maybe i have to?
any info on recrystalization or eluent mixtures for flash/tlc chromatography would be great.
thanks!
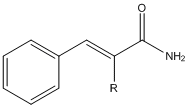
EDIT : P.S. in the image below R=H or alkyl groups, (Cinnamamide - R=H, alpha-ethylcinnamamide - R=ethyl)
[Edited on 25-2-2009 by Isomeric_Fred]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
It seems obvious you are trying to work up a reaction mixture from a Heck coupling, so first of all filter it trough celite or silica to remove the
catalyst, then dilute with 4-5 volumes of water, remove bromobenzene remains and other water insoluble volatiles with steam, then cool and separate
the insoluble material (it is possible that at this point the product will crystallize, but more probably it will just be viscous oil or a resin-like
stuff) and recrystallize it (from H2O/EtOH or check the literature on what solvent is best for cinnamamide).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Isomeric_Fred
Harmless

Posts: 31
Registered: 25-6-2006
Member Is Offline
Mood: curious
|
|
Hi Nicodem and thanks 
Yes I am carrying out heck couplings. celite aid filtration is a must here since the catalyst ends up very fine and will clog filter paper. i havent
diluted with water though, and havent thought about it. good idea. will give it a go and report back here.
if anyone has any other idea plz share 
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Isomeric_Fred, could you give us a little detail on the reaction? I'm pretty interested in Heck-like couplings...
What catalyst did you use? The tetrakis(triphenylphosphine)palladium? Did you prepare it before hand or in-situ? I suppose you used dimethylacetamide
as solvent...
I'd love to hear more about the way the reaction proceeded and the results you obtained!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Isomeric_Fred
Harmless

Posts: 31
Registered: 25-6-2006
Member Is Offline
Mood: curious
|
|
Hi Klute. I havent carried out the reaction with acrylamide yet, since I wasnt sure about how to purify efficiently the cinnamamide.
I have carried a heck coupling of phenylbromide and acrylate ester in NMP and NaOAc as base and 0.1mol% of Pd(OAc)2 as catalyst with no ligand. Ive
let it react for 6Hours at 130-140degC. have gotten very good yields, very similair to that described in the journal article describing this
methodology, albeit somewhat lower - around 85-90% - still very acceptable i think 
I really like this heck methodology. No need for expensive or sensitive phosphines. also very low catalyst loading makes this approach really alot
cheaper then traditional heck.
Im sure its not suitable for all substrates, but it seems rather general and good yielding in most circumstances. Still havent had the chance to test
it with unprotected acrylamide, but i think it will work. dont see any reason why it should, hopefully the amidic nitrogen wont complex with anything.
I think the first publication of the sort (dont flame me if its not the first one) is "Homeopathic Ligand-Free Palladium as a Catalyst in the Heck
Reaction. A Comparison with a Palladacycle", Andre H. M. de Vries,* Jan M. C. A. Mulders, John H. M. Mommers, Huub J. W. Henderickx, and Johannes G.
de Vries*. ORGANIC LETTERS 2003 Vol. 5, No. 18 3285-3288 (attached, along with the experimental).
The above reaction details ive layed out are described in that article which ive followed closely.
The authors say at the end of the journal that they've scaled up this procedure to 1Kg scale, which is very encouraging, and next time ill test it ill
test it with 1ml of solvent per 1mmol of substrate, instead of the ratios given by the article (which are for small scale, they dont give experimental
details of scaled up reaction).
Attachment: palladium acetate heck - de. vries.pdf (73kB)
This file has been downloaded 773 times
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
| Quote: | Originally posted by Isomeric_Fred
The m.p. of cinnamamide is around 140ish degC so distilling is out of the questions.
|
Fred- why can't you pull a hard vacuum on it and distill it out? I've done this with cmpds that melted at 150-60..
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thank you very much for the details and the article! That is a reaction I would love to try one day...
How did the workup go for your reaction?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|