| Pages:
1
2 |
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
a phenylpropyloxy ether
OK, I can start again, and ask in the best way I know how. I'd like to construct a phenylpropyloxy ether using the alcohol, and was imagining I’d
need 3-bromopropylbenzene to perform the alkylation (the ether would be the leaving group?), and HBr off the other end. So, I would imagine
propylbenzene would be the 2ndary precursor (to 3-bromopropylbenzene, the primary), but I know this simple aromatic can also be used to make a
controlled substance P2P, so I am leary of ordering it. Maybe someone has some helpful ideas on how I could accomplish this particular reaction
without alarming anyone? Honestly, I could care less about amphetamines. It would just seem such a waste of time to have to synthesize such a simple
chemical, propylbenzene. Is there an easier way to perform this alkylation? Thanks
[Edited on 9-2-2009 by Globey]
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
http://www.freechemsketch.com/
If you are having a hard time explaining what you want, as I noticed in your other thread, download chemsketch and draw the reaction or the molecule
you have in mind.
Just an Idea 
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Is this close to what your attempting to accomplish?
[Edited on 10-2-2009 by Sedit]

|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sedit
................
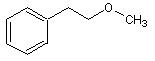
Is this close to what your attempting to accomplish?
[Edited on 9-2-2009 by Sedit] |
Sedit,
sry, don't see anything but some banner saying "remote linking prohibited"
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Really sorry I didnt know that the picture shows up on my end.. How do I go about adding a picture then?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sedit
Really sorry I didnt know that the picture shows up on my end.. How do I go about adding a picture then? |
Working for me, but the best way is to save the image on your computer, then use the "attachment" dialog at the bottom of the posting edit stuff to
browse for that file.
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
Sedit, that would be it. Hopefully I more accurately described the problem: forming phenylpropyloxy ether using an alcohol. I read in Arctander
that P2P actually could be reduced to propylbenzene, but that seems an awful lot of trouble to go through to get the desired molecule (and illegal to
do without the proper license.) 1-Phenyl-2-nitropropene is not illegal, per se. Could it be used to go directly to propylbenzene? Also, was I right
that I'll need something like bromoisocumene to do this, or might there be another way? Tnx
[Edited on 10-2-2009 by Globey]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
You'll get a lot further if you just ask your questions in a straightforward and clear way and stop talking about controlled substances and legality.
It adds nothing to the substance of your inquiry. If you can't discuss such a simple molecule without constantly mentioning its nefarious relationship
to other molecules that you wish to avoid, it gives precisely the opposite idea about your intentions.
PGP Key and corresponding e-mail address
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I read a comment in the locked thread you started that suggested you work on communication skills. I second that wholeheartedly. I had written a
reply to what I thought you were asking about and just now deleted it. Work on the language so we can understand what it is you're talking about.
And as the man says, leave the legallities alone. If you're trying to make p2p some backassward way it will be obvious and the thread will be locked
again. There are plenty of places on the net you can find out how to do that. It's not very interesting chemistry as a whole and not at all what
this place is about.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
| Quote: | Originally posted by Globey
Sedit, that would be it. Hopefully I more accurately described the problem: forming phenylpropyloxy ether using an alcohol. I read in Arctander
that P2P actually could be reduced to propylbenzene, but that seems an awful lot of trouble to go through to get the desired molecule (and illegal to
do without the proper license.) 1-Phenyl-2-nitropropene is not illegal, per se. Could it be used to go directly to propylbenzene? Also, was I right
that I'll need something like bromoisocumene to do this, or might there be another way? Tnx
[Edited on 10-2-2009 by Globey] |
Ok see that a pictures worth a thousand words....two thousand in your case
Why does it seem as though you are trying to make an ether synthesis into so many unnessasary steps?
Have you ever considered dehydration of the corresponding alcohols?
This will however prove to be problematic due to the formation of other side products but mainly just a tool for you to visualize ways of going about
this without propylene benzene.
[Edited on 10-2-2009 by Sedit]

|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I still do not know what the question is. Globey, up to now you wrote only a single sentence that only roughly indicates what your problem might be.
Apparently you want to do a Williamson ether synthesis using phenylpropylbromide (which one? there are 6 isomers of which 3 aliphatic bromides useful for such a reaction).
I highly suggest you to take Polverone's suggestion seriously. When someone spends only one sentence on explaining his question and whole paragraphs
on talking about precursors to illegal substances it is obvious he is either a highly confused person or has some nefarious business in mind (or most
likely both). In any case this is not tolerated here and will upset a whole lot of members. Also, when opening threads without providing a single
reference or without properly explaining the topic, please do so in the Beginnings section where I'm moving this anyway.
Sedit, the ether in your first picture is a 2-phenylethyl one, not any of the phenylpropyl ones.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Phenyl magnesium halide + 1,3 propylene oxide ==> 1-phenyl-3-hydroxy-propane.
That is used in a Williamson ether synthesis with a methyl halide, tosylate, or what have you, to give the methyl ether. Keeping the propanol as
alcohol reduces problems with elimination forming the alkene.
It might be possible to use the magnesium halo-alkoxide from the Grignard+epoxide reaction in the Williamson.
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
not_important: You both understood, and answered my question. Many thanks The
alcohol in question is otherwise labile, so the (above) scheme seems additionally elegant. Yup, I would go directly with the intermediate from the
Grignard (not isolating). Wouldn't be so worried about forming a dbl. bond on the chain as I am about destroying the sensitive parts of the alcohol,
but I suppose that is a concern in your typical ether (dehydration) synthesis. Thanks for the help The
alcohol in question is otherwise labile, so the (above) scheme seems additionally elegant. Yup, I would go directly with the intermediate from the
Grignard (not isolating). Wouldn't be so worried about forming a dbl. bond on the chain as I am about destroying the sensitive parts of the alcohol,
but I suppose that is a concern in your typical ether (dehydration) synthesis. Thanks for the help
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by not_important
Phenyl magnesium halide + 1,3 propylene oxide ==> 1-phenyl-3-hydroxy-propane. |
Interestingly enough, oxetan (1,3 propylene oxide) can also be used in a Friedel-Crafts reaction with benzene to give the same product (JACS,
76 (1954) 2313-2314) in 71% yield, while the use of PhMgX with CuI as catalyst gives 52% yield (Tetrahedron Lett. (1979) 1503-1506).
| Quote: | Originally posted by Globey
The alcohol in question is otherwise labile, so the (above) scheme seems additionally elegant. Yup, I would go directly with the intermediate from the
Grignard (not isolating). |
It only seems elegant because you haven't bothered to check the price of oxetan, which happens to be approximately 600-times more expensive than
3-phenylpropanol (which is ~50EUR/kg only). I hope that by "alcohol in question is otherwise labile" you don't mean 3-phenylpropanol, since this is
anything but labile.
I have not understood the rest of your post so I can't comment, except repeat that you really, really should work on your communication skills.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
| Quote: | | Sedit, the ether in your first picture is a 2-phenylethyl one, not any of the phenylpropyl ones. |
Yes your absolutly correct. Sorry about that I will fix it Asap.
~Sedit
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@NIcodem: which is more commonly used terminology, "phenylethyl" or "phenethyl"? I've seen both and am not sure which to use in searches at ACS, RSC
or patent finder.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
@CRX: "phenethyl" I commonly encounter more in the older literature. But there is no harm in doing searches for both "phenethyl" &
"2-phenylethyl". 
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
| Quote: |
It only seems elegant because you haven't bothered to check the price of oxetan, which happens to be approximately 600-times more expensive than
3-phenylpropanol (which is ~50EUR/kg only). I hope that by "alcohol in question is otherwise labile" you don't mean 3-phenylpropanol, since this is
anything but labile.
I have not understood the rest of your post so I can't comment, except repeat that you really, really should work on your communication skills.
|
Generic examples here were meant to illustrate general concepts. No one said that the prototype example reflected the actual reaction.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Google ether synthesis. It brings up about 3 different synthesis on the first page alone although for you I feel Williamson synthesis to be your best
way to go about it because something tells me that magnesium halides are going to be problematic for you.
| Quote: | | No one said that the prototype example reflected the actual reaction. |
please do us all a favor and post the actual reaction reaction the so we dont spend days playing guessing games.
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
Snip... | Quote: | Originally posted by Sedit
something tells me that magnesium halides are going to be problematic for you. | Quote: |
I've never had trouble under less than anhydrous conditions. And I'm not just talking a small seed of iodine, trick. Did you think you were clever
with that insult? Best to choose the high road, if ya know what I mean Best to choose the high road, if ya know what I mean |
|
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
LOL touchy arn't you? 
No I was trying to help you but if its unwanted I will just as well leave this threed of confusing rhetoric alone. Sorry to say but you have shown
that you havent slightest Idea of simple ether synthesis and your telling me that the grignard is simple?
So no insult was intended but Im sure i could think of one if thats what you wish!
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sedit
LOL touchy arn't you? 
No I was trying to help you but if its unwanted I will just as well leave this threed of confusing rhetoric alone. Sorry to say but you have shown
that you havent slightest Idea of simple ether synthesis and your telling me that the grignard is simple?
So no insult was intended but Im sure i could think of one if thats what you wish! |
You know what, I think your last response was tailored to my last reply. Sort of like the person who takes a joke offensively, and then the joke
teller says "just kidding, tjeeze! (as if they are the one who is offended)", but only after the response (but of course). I guess I must have the
"dumb" sticker still stuck to parts of me...no offense Now, if you want to have
serious chemistry talk without personalizing things, I suggest you stop taking low ball stabs, and (now) start being honest. Now, if you want to have
serious chemistry talk without personalizing things, I suggest you stop taking low ball stabs, and (now) start being honest.
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Try alkylating of benzylgringard with methyl cellosolve tosylate, mesylate etc (methyl cellosolve should be a solvent), phenethyl gringard with
methoxymethyl chloride(made from HCl, CH2O and MeOH)
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
^^^^^^^^is that supposed to be funny? hehehe
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
yes, because benzylchlorides suppose to be lachrymators))
by the way, why not to start from either 3-phenylpropanol(that Nicodem suggested) or cinnamic acid?
[Edited on 11-2-2009 by Ebao-lu]
|
|
|
| Pages:
1
2 |