| Pages:
1
2 |
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Interesting energetic materials
I recently borrowed "Reactive Chemical Hazards" from my library. The book being over 1600 pages was almost completely comprised of
explosives, front to back cover. So, I went through it, almost every single page looking for things to catch my eye, these are sum of the highlights.
Some may have been discussed before many are totally impractical to study, but what the hey, I'm hoping to inspire here and this is a
sciencemadness board after all!
| Quote: |
3 - Nitroperchlorylbenzene
O2NC6H4ClO3
"McCoy, G., Chem. Eng. News, 1960, 38(4), 62"
The nitration product of perchloryl benzene is explosive, comparable in shock-sensitivity with lead azide with a very high propagation rate.
1,1,1,3,5,5,5 - Heptanitropentane
(O2N)3CCH2CH(NO2)CH2C(NO2)3
"Klager, K. et al., Propellants, Explos., Pyrotech., 1983, 8, 25-28"
This explosive compound has a +12% oxygen balance so can function as an oxidant.
1,1-Diazidoethane
(N3)2CHCH3
"Forster, M. O. et al., J. Chem. Soc., 1908, 93, 1070"
The extreme instability and explosive behavior of this diazide caused work on other gem-diazides to be abandoned.
Aluminum tetraazidoborate
Al[B(N3)4]3 or AlB3N36
"Mellor, 1967, Vol. 8, Suppl. 2, 2"
A very shock sensitive explosive, containing nearly 90 wt% of nitrogen.
|
Also, an interesting note on tetranitromethane, "When mixed with hydrocarbons in approximately stoicheiometric proportion a sensitive highly
explosive mixture is formed.... explosion of only 10 g of a mixture with toluene caused 10 deaths and severe injuries..." jeeze, that sounds like
quite the accident.
E. by Chemo: title
[Edited on 9-8-2005 by chemoleo]
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
for 1,1,1,3,5,5,5 - Heptanitropentane see mega's site
Al tetraazidoborate would disperse <font color=red>hot</font> B & Al into air upon detonation making a dazzling fireball
Edit: not the pyrophoric metal but the very stable nitrides BN and AlN
[Edited on 8-12-2003 by KABOOOM(pyrojustforfun)]
|
|
|
cumbustion
Harmless

Posts: 21
Registered: 7-8-2005
Member Is Offline
Mood: what the hell k
|
|
What of their preparations?
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
12 azide groups per molecule!? I'm sure that'll get some people here drooling!
|
|
|
Swany
Hazard to Others
  
Posts: 188
Registered: 11-4-2005
Location: My happy place...
Member Is Offline
Mood: Sanguine
|
|
How about XeO3? The first oxide explosive developed in 1962. XeF6 and XeF combonations(white solids) were left in water and XeO3 formed accidently.
Naturally it is quite unstable and it took a while to solve the puzzling decomposition of what were assumed to be xenon flurides.
To make XeF6, XeF4, and XeF8, you react flurine and xenon in a nickel crucible at 400C. For more information see The Noble Gasses by Isaac Asimov. It
is an interesting read.
The preperation of Xe compounds seems to be true sciencemadness material.
|
|
|
praseodym
Hazard to Others
  
Posts: 137
Registered: 25-7-2005
Location: Schwarzschild Radius
Member Is Offline
Mood: crazy
|
|
But they have relatively low melting points.
This link here has more information on xenon compounds.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Mellor's did not give a reference unless it is in an earlier paragraph. It appears that the cpd is made from aluminum borohydride Al(BH4)3 and
HN3. Al borohydride is made from other borohydrides and an Al halide, but is nasty stuff itself. A preparation of the borohydride is here.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Ethylammonium tetranitratozincate
(EtNH3+)2 Zn(NO3)4 -2
Zn(NO3)2 + EtNH2 in liq. N2O4 solution
C.C. Addison & N. Hodge, JCS 1954, 1138-43 & CA 48, 7477 (1954)
N1,N6-(Ditetrazolyl-5)-hexazadiene<pre>
N-NH-C=N-N=N-N=N-N=C-NH-N
|| | | ||
N----NH HN---N</pre>
The ends are rings, if you can't interpret the above poorly shown structure.
Explodes when rubbed
N content= 87.49%
Prepared by diazotization of 5-amino tetrazole, followed by treatement with an aq. solution of hydrazine hydrochloride in the presence of sodium
acetate at low temp.
Forms explosive salts.
Not sure what references give the synthesis, check The Encyclopedia of explosives Vol 1 pg A260.
Other interesting explosives are ammonium and hydrazine salts of oxidizers, for example ammonium permangante and ammonium dichromate. Hydrazine salts
of those acids are probably also explosive.
|
|
|
Madandcrazy
Hazard to Others
  
Posts: 117
Registered: 11-5-2005
Member Is Offline
Mood: annoyed
|
|
Interesting thread.
N1,N6-(Ditetrazolyl-5)-hexazadiene more a fantastic stuff than it exist in realy ?
Listed materials are probably instable.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
No, surprisingly it does actually exist, but it is very sensitive. I will attempt a preperation when/if I get some 5-amino tetrazole prepared for
some other experiments I want to try. I only found out about the tetrazole ring structure a few days ago but I find it quite interesting, so of
course I am going to make some compounds containing it.
|
|
|
DeAdFX
Hazard to Others
  
Posts: 339
Registered: 1-7-2005
Location: Brothel
Member Is Offline
Mood: @%&$ing hardcore baby
|
|
http://www.intdetsymp.org/detsymp2002/PaperSubmit/FinalManus...
Hmm... This document compares the effects of Nanometer Aluminum against Micrometer Aluminum in Ammonium Nitrate, TNT/RDX, and PBX based explosives.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Unless the aluminum has been in an inert atmosphere all the time
as well as when mixed, it will have a surface oxide layer. This
probably has the single most contribution to reactivity or lack
of, with this material. While particle size will matter, I really
doubt it makes a difference to have colloid sized particles as
opposed to suspension sized. The added detraction is that the
greater surface area means that more alumina / oxide is present
and therefore contributes more inert matter to interfere with
detonation. There probably exists a happy median as to ideal
particle size but this author does not delve into answering
the question.
As an alternative to buying expensive ready made reagents be aware
Calcium is dissolved entirely by anhydrous ammonia, which upon
drying percipitates as the elemental pyrophoric powder. Larger
particle size is used for thermate and flash compositions however.
.
|
|
|
SilencePlease...
Harmless

Posts: 5
Registered: 12-8-2008
Member Is Offline
Mood: No Mood
|
|
I regret to inform that my pdf with B(N3)4 salts has gone missing....
"They" made it by reacting HN3 with a R-NH-B(N3)2:
R-NH-B(N3)2 + 2HN3 > R-NH3.B(N3)4
I can recall R being 2,2,6,6-tetramethylcyclohexane.
|
|
|
F2Chemist
Harmless

Posts: 28
Registered: 30-6-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Swany
How about XeO3? The first oxide explosive developed in 1962. XeF6 and XeF combonations(white solids) were left in water and XeO3 formed accidently.
Naturally it is quite unstable and it took a while to solve the puzzling decomposition of what were assumed to be xenon flurides.
To make XeF6, XeF4, and XeF8, you react flurine and xenon in a nickel crucible at 400C. For more information see The Noble Gasses by Isaac Asimov. It
is an interesting read.
The preperation of Xe compounds seems to be true sciencemadness material. |
XeO3 is simply the oxidation of XeF4. XeF4 is easily prepared by reacting Xe and F2 at high temperatures. It is also a minor contaminant when XeF2
is prepared using UV light (Xe + F2 + UV light). This used to be a big problem with the preparation of XeF2...trace amounts of XeF4 were made,
leading to an explosive mixture.
XeF8 does not exist. It's reported synthesis was later disproved.
XeF2 is a beautiful compound...nice white crystals.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Xenon(VIII) tetroxide, XeO4, also exists, as a crystalline solid, obtained by the action of O3 or atomic oxygen on XeO3 or xenates(VI). There may also
be an (VIII) oxyfluoride, XeO3F2. Although the (VIII) compounds, comprising XeO4 and perxenates XeO6--, are the most stable Xe compounds, they can
still be dangerously explosive.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Ozobenzene:
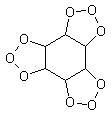
made by leading dry O3 into pure benzene for 10 to 12 hours at the highest 10º. It is an amorphous powder. Deflagrates if quickly heated at 50ºC.
Explodes very violently from friction or pouring conc. H2SO4 or KOH solution over it. Warm water contact is also said to cause explosion. Water
decomposes it immediatley to CO2, formic and acetic acids. Insoluble in alcohol, ether, CHCl3, CS2, and ligroin. Contact with ice water is said to
form a crystalline material which explodes at the slightest touch. There is a reference of Scientific American Monthly 1, 144 (1920) stating it has a
higher VOD than ordinary explosives. Its reported heat of explosion is 2000 cal/g, so using an estimated density of 1.78 g/cc, VOD is around 9452 m/s.
Dicyanogen-di-N-oxide:
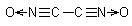
crystalline solid is stable at -78ºC for several hours, it decomposes spontaneously at -45ºC in a vacuum without explosion emitting a white light,
at atmospheric pressure and brought to room temperature, it detonates. It is made by treating dichloroglyoxime with Na2CO3 solution, this needs a more
complicated work-up, dilution and cooling with solvents to avoid explosion.
Tetraacetylenedicarboxylic acid:
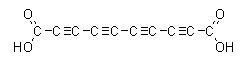
this is an extraordinarily explosive crystalline solid, and is decomposed by light turning red, then black. This black mass explodes when heated.
Diazotetrazole:
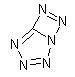
The free tetrazole is highly unstable, explosive and decomposes from heating by boiling in water basically to cyanogen and N2. It is known to explode
in solution when the concentration reaches about 1%, but its salts are much more stable.
Sodium oxyazotetrazole: Na2C2N10O: the pentahydrate are yellow leaflets which explode exceedingly violently when heated.
Potassium pentoxime: K2C5H3N5O5: obtained as exceedingly explosive brown-yellow flakes by solubilizing pentoxime in
KOH and then precipitating with alcohol.
Acetoethyl nitrate (aldehydenitric ether). C2H4O.2(C2H5NO3): made by dry distillation of saltpeter with potassium ethylsulfate. It is
a spicy smelling, sweet tasting liquid. Bp: 84-86 deg., D = 1.0451 at 19 deg. Explodes violently it heated above its boiling point. Insoluble in water
and decomposes when warmed in KOH solution to aldehyde and KNO3.
[Edited on 17-8-2008 by Schockwave]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Here's another drawing of 1,6-Di(tetrazol-5-yl)hexazadiene:
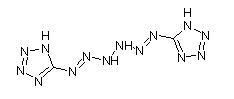
And then, here's an interesting document on a few related compounds, with discussion of some newer materials.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
A compound which might have the formula: CH3.C(OH)2.C(N.OH).C(N.OH).CO.O.C(OH.CH3).C(N.OH).C(N.OH).CO2H = C10H14N4O11 (Beilstein I 601) made
by leading in N2O3 into ethereal solution of several times and freshly distilled levulinic acid (pure, crystallized levulinic acid doesn't form it).
It is an amorphous powder being insoluble in cold solvents, and decomposes by heating. No idea on other properties, but it is said to explode by
addition to H2SO4.
Acetyl nitrite: C2H3O3N, made from addition of NOCl onto silver acetate which is cooled with a freezing mixture. It's an unstable,
gold-yellow liquid which decomposes in direct light, slower in diffused light, forming acetic anhydride and N2O3. Its vapor explodes violently on mild
heating.
Alkyl nitrites: can be explosive, ethyl nitrite (can expl at about 90°C, Bp. 17.4°C), pentyl nitrite (expl heating above 250°C),
and allyl nitrite (vapor is known to expl at 100ºC). But especially the gas (Bp. -16° C) methyl nitrite, which heat of formation gets us a TNT
equivalence estimate of 122 to 132%.
Silver azidotetrazole: AgCN7, flakes which explode extraordinarily violently when warmed under water, or by contact in a moist
condition.
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by Schockwave
Here's another drawing of 1,6-Di(tetrazol-5-yl)hexazadiene:

And then, here's an interesting document on a few related compounds, with discussion of some newer materials. |
This compound is well known, it's powerfull and extremely sensitive exposive. Can be made from hydrazine and diazotetrazole solution. I was once
prepared sample of this substance it is star like yellowish crystalls, very violently exploding on small eternal stimulus. Compound could also form
salts, and is slowly decomposed by water. Too dangerous to handle (on level of tetrazylazide), but extremely brisant.
Here is description of this substance from my tetrazole article:
3. Bis-diazotetrazolylhydrazine (H(CN4)-N=N-NH-)2 – yellowish crystaline plates, with characteric star like edges. Poorly soluble in water and
organic solvents. Due to the high nitrogen content (14 nitrogen atoms and only 2 carbon atoms), the substance is an extremely powerful explosive. Pure
product, thoroughly washed by ethanol melts at 120-123°С, where as the crude product explodes with terrible violence when heated to
90°С. Quite stable at room temperature, but very sensitive to friction and impact, violently explodes when rubbed with a glass rod or spatula.
The action of alkaline solutions result in fast decomposition to 5-azidotetrazole, 5-aminotetrazole, nitrogen, ammonia and dicyan. Slowly decomposed
when stored underwater due to hydrolysis. Can form extremely explosive salts, if water suspension of bis-diazotetrazolylhydrazine at 0°С is
treated with a 20% solution of copper sulphate, greenish crystalls of copper salt are formed. Copper salt is extremely sensitive to friction, impact
and fire, flash point 185°С. Bis-diazotetrazolylhydrazine is one of the richest on nitrogen from all known organic substances, containing 87.5%
nitrogen.
Synthesis of bis-diazotetrazolylhydrazine: 0.75g of hydrazine chloride and 1.5g of sodium acetate are dissolved in 3 ml of water, solution is cooled
in a sodium chloride/ice bath and a solution of 1.9g diazotetrazole (Note №1) is slowly added with stirring. The yellow amorphous precipitate is
washed with ice cold water, alcohol, and ether and is dried at room temperature. This work requires the greatest caution, due the extremely explosive
nature of synthesized substance.
Notes:
1. Solutions of diazotetrazole are extremely explosive and can explode spontaneously, so appropriate safety measures should be taken into account all
the times. It’s not recommended to make amounts bigger than those presented in the synthesis procedure due to safety reasons. All works should
be carried out by experienced chemist, with the greatest caution.
[1] P.F. Bubnov “Primary Explosives And Initiation Devices” Part 1, Moscow, Ministry of Defence Industry Press (1940), pp 313-314.
[2] L.I. Bagal “Chemistry And Technology of Primary Explosives”, Moscow, 1975, pp 399.
[3] L.I. Khmelnitskij “Handbook Of Explosive Materials” Part 2, Moscow, 1961, p 96.
[4] Patents DE362433C1, DE400814C1.
[Edited on 27-8-2008 by Engager]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by Schockwave
Diazotetrazole:

The free tetrazole is highly unstable, explosive and decomposes from heating by boiling in water basically to cyanogen and N2. It is known to explode
in solution when the concentration reaches about 1%, but its salts are much more stable.
Sodium oxyazotetrazole: Na2C2N10O: the pentahydrate are yellow leaflets which explode exceedingly violently when heated.
|
Here are descriptions of both substances from my tetrazole article:
Diazotetrazole
Diazotetrazole was first prepared by Thiele during the diazotation of 5-aminotetrazole with sodium nitrite in hydrochloric acid, but he failed to
indentify reaction product, because at 0°С it exploded in solution. Later it was shown that the product of this reaction is the extremely
unstable diazotetrazole. Diazotetrazole is an extremely sensitive and unstable explosive, 6-7% solutions can explode spontaneously even at 0°С,
breaking the glassware used for the diazotation. In acidic environment diazotetrazole forms ions of tetrazolediazonum, which are combined with acid
anion to form the diazonium salt. Tetrazole diazonium salt is also very unstable and its behavior resembles that of free diazotetrazole. In a basic
environment diazotetrazole forms anions of tetrozoliumdiazotate, which are combined with base cations to form stable salts, readily soluble in water,
and explosive in the dry state.
The extremely unstable nature if diazotetrazole and it’s diazonium salts is almost unprecedented for known chemical compounds, it can explode
even in 1% solutions. The most likely reason for this instability is the strong electron withdrawing effect of the diazogroup, which lowers the
stability of tetrazole ring and lowers the activation energy for decomposition to a neglible level. Stability of tetrazoliumdiazotate salts can be
explained by the electron saturation of diazogroup by hydroxide ions, oxygen atom on the end of a diazogroup lowers its electron withdrawing ability
and stabilizes the tetrazole ring. Tetrazolediazotate salts are so stable, what they can be simply prepared in free state.
Tetrazoliumdiazotate can exist in two isomeric forms; cis and trans form. Usualy the unstable cis form is formed first, and transforms to stable trans
form by long boiling, reverse transformation takes place only by action of ultraviolet radiation.
The difference in stability of two forms is so great, that they can be practically be termed as different substances, because of the large difference
in physical properties. For example the pale yellow or colorless trans diazotate salts are quite stable in the dry state, but the cis diazotate salts
are unstable and explosive in dry state even with stabilizing aromatic rings. During acidification the bifunctional trans-diazotate anions transform
to trans-diazotetrazole hydroxide or to N-nitrosoamine, which are further transformed to the cation of tetrazolediazonium. Formation of nitrosoamine
can be easily noted by green coloration of solution, usual for nitrosoamines. Freshly prepared diazotetrazole solutions are always slightly green
colored.
The Sodium salt of trans-tetrazolium diazotate Na(N4C)-N=N-ONa, exists as white needle-like crystals, is very soluble in water, and weakly soluble in
ethanol. On heating the salt deflagrates without melting, solutions of the salt is acidic due to the hydrolysis. In a neutral or weakly acidic
environment sodium salt solution reacts with lead salts, forming the lead salt of oxyazotetrazole, insoluble in water, alcohol and either, but soluble
in hydrochloric acid, sodium hydroxide and ammonia. Salt deflagrates at 360°С. Barium salt of tetrazoliumdiazotate BaCON6 – yellow
crystalls, readily soluble in water.
Diazotetrazole synthesis: 5g of aminotetrazole is dissolved in 30 ml of water, then 6 ml of 25% sodium hydroxide and 3.4g of sodium nitrite are added.
After the nitrite is completely dissolved the mixture is cooled in an ice bath, placed in dropping funnel and is added slowly in 10-12 minutes with
efficient stirring and cooling by ice cold water to mixture of 16 ml of 30% HCl with 170g of ice. Temperature of the reaction mixture should be below
or at 0°С all times (Note №1). In the end of reaction mixture has slightly green color, because of presence of equlibrium quantities of
nitrosoamine. Diazotetrazole solution is best used imidately, if that is imposible, it should be imidately transformed to tetrazolium diazotate by
treatment with solution of sodium hyroxide (Note №2).
Notes:
1. Diazotetrazole is an extremely sensitive explosive, it’s water solutions at concentrations about 6-7% explode spontaneously even at
0°С, breaking up glassware used for diazotation. According to some sources at 0°С even 2% solutions can explode.
2. Because of the extreme unstable nature of diazotetrazole, it’s usually produced in the form of 2-3% solutions with use of appropriate safety
procedures (use of shields), and if it is not needed for further synthesis, diazotetrazole must be imidately transformed into tetrazolium diazotate
salt by action of 25 ml of 25% sodium hydroxide (until basic reaction). If diazotetrazole is needed for further synthesis, the solution of tetrazolium
diazotate salt is cooled to 0°С and is activated by addition of corresponding amount of hydrochloric acid.
[1] Justus Liebig's Annalen der Chemie, Volume 273, Issue 2-3, pp 144-160 (1893) ; Johannes Thiele, J. T. Marais
“Tetrazolderivate aus Diazotetrazotsäure”.
[2] P.F. Bubnov “Primary Explosives And Initiation Devices” part 1, Moscow, Ministry of Defence Industry Press (1940), pp 311-312.
Sodium oxyazotetrazole
Sodium salt of oxyazotetrazole Na2C2N10O*5H2O. Then sodium tetrazolium diazotate solution in fairly concentrated solution is boiled for long time, or
it’s warm solution is treated with stream of CO2, sodium salt of diazotetrazole trans-forms to sodium salt of oxyazotetrazole, precipitated from
solution because of moderate solubility in water. Salt can be easily differed from diazotate by its characteristic dark-yellow color. Mechanism of
this reaction is unknown, but it is most possible it is the same as formation of oxyazobenzene from benzolediazonium-nitrate and barium carbonate. The
sodium salt of oxyazotetrazole exists as yellow crystaline plates, relatively insensitive to friction or impact, but exploding with terrible violence
on heating. The barium salt of oxyazotetrazole is also known, it is a yellow compound crystallizing into a needle shape with 4 molecules of water. On
prolonged boiling the salt slowly decomposes with formation of gaseous products, attempts to isolate free acid by action by action of strong acid
solution on sodium salt resulted in formation of a solution, containing an unidentified substance which readily decomposed in solution. Structure of
substance was not discovered because of very small amounts were available for analysis.
[1] Justus Liebig's Annalen der Chemie, Volume 273, Issue 2-3, pp 144-160 (1893) ; Johannes Thiele, J. T. Marais
“Tetrazolderivate aus Diazotetrazotsäure”.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
That's good stuff Engager. Lots of interesting chemistry in high nitrogen compounds.
Has anyone information and properties of acyl perchlorates, namely acetyl perchlorate? All I've been able to find its use in polymerization or as a
catalyst, etc.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Another compound worth mentioning, knowing how SM likes the tetrazoles, which compound in question is 5-Cyano-(a-tetrazole);
tetrazole-5-carbonylonitrile (5-CNTZ) described shortly in Federoff C588-9. This substance is an already quite known energetics precursor itself. The
silver salt, AgC2N5 is known to explode on heating. Barium salt is also known Ba(C2N5)2.3 1/2 H2O. Chemical Abstracts, Vol. 13, p. 708-710 from here mentions in addition to this and other items, the the silver salt (formula should be Ag2C4N12) of ditetrazyltetrazine (formed from
cyanotetrazole and hydrazine, then EtNO2) which is described as a dark violet precipitate which explodes at 150 deg.

|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
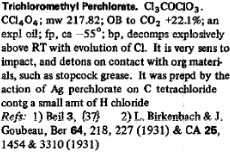
Then also trichloromethyl perchlorate which can be formed from AgClO4 and CCl4.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Higher density formulations
This peaked my interest only because the procedure is similar to that which
may be applied to the synthesis of tetranitromethane. I'm wondering out loud
so to speak, if the perchlorate analog methane could be realizable. C(ClO4)4
I have mentioned before the observation that density factors very significantly
not only in molecular compounds but to fuels and oxidizers in terms of energy
density. This is most salient in the fact that there is more hydrogen in a given
volume of gasoline than the equivalent volume of liquid hydrogen. The naive
notion that the pure elements reacting will provide the highest possible energy
density for an amount of material is evidently wrong. Not so well understood
is the fact that compounds particularly of trinitromethane similarly contain
more oxygen per given volume than the equivalent amount of liquid oxygen !
Download 2 file zip _ http://ifile.it/uwgtcvx
A further enhancement which might be possible for (bis)2,2,2-trinitroethyl-carbonate
would be to substitute the carbonate functional group with something more
energetic. The acid H2XeO4 formed by the hydrolysis of XeF6 allows the
application of a xenate XeO4= functional group. The result is a very energetic
explosive superrich in oxygen that can be exploited with strongly endothermic
fuels such as for example calcium carbide with an expectedly high performance.
We already know that ethyl perchlorate is exceedingly powerful but impractical
for it's sensitivity. I wonder also how a perchlorate of a 2,2,2-trintroethyl goup
might compare, the density is key, if it provides no advantage then there is
no point to it.
- For reasons unknown it remains insuperable to download a zip file from
the forum server that can be opened without producing a CRC error.
.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
This peaked my interest only because the procedure is similar to that which
may be applied to the synthesis of tetranitromethane. I'm wondering out loud
so to speak, if the perchlorate analog methane could be realizable. C(ClO4)4
(cut). |
Carbon tetraperchlorate, or tetraperchlorylmethane, if it could be made, is NOT analogous to
tetranitromethane, C(NO2)4, because in the former the bonds are via oxygen, C(-O-ClO3)4, i.e. a covalent ester, whereas in the latter the bonds are
C(-NO2)4. However, I doubt that it has been synthesized; I wonder if it could be done by forcing CO2 under great pressure into concentrated HClO4 or
liquid Cl2O7.
The analogous nitrogen-containing ester (to the perchlorate ester) would be carbon tetranitrate, or tetranitrylmethane, C(NO3)4, with bonding via
oxygen, C(-O-NO2)4; I doubt that it has ever been synthesized. I wonder if it could be made e.g. by forcing CO2 under great pressure into concentrated
or fuming HNO3, or by the action of liquid CO2 under pressure on N2O5 (which however in the solid state is nitronium nitrate, meaning that formation
of the nitro-compound via the NO2+ cation is more likely).
|
|
|
| Pages:
1
2 |
|