trinitrotoluene
Hazard to Others
  
Posts: 142
Registered: 17-10-2002
Location: California
Member Is Offline
Mood: paranoid
|
|
Zinc Sulfide
I had heard about zinc sulfide as a chemical that glows, it is activated with copper and silver using high heat.
Will this reaction toward making the sulfide work, which is mix zinc powder with sulfur and burn it, will the leftover product be zinc sulfide, ZnS?
TNT
|
|
|
chloric1
International Hazard
    
Posts: 1147
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Hmmm
Most inorganic luminescent materials usually need to be calcined for many hours or even days anywhere from 600 to 1300 C.  Have a kiln? Consult the US patent office at uspto.gov and do a search for fluorescent
or luminous inorganic pigments. The europium doped alkaline earth aluminates fascinate me most. Have a kiln? Consult the US patent office at uspto.gov and do a search for fluorescent
or luminous inorganic pigments. The europium doped alkaline earth aluminates fascinate me most.
[Edited on 11/23/2003 by chloric1]
Fellow molecular manipulator
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
impure ZnS is phosphor(means it has phosphorscence property) may be it has some effects on deflagration scene of the Zn-S mix. the magically beautiful
brilliant green fireball.
copper presence? hmm.. what about a thermit like reaction between copper polysulfide and zinc?
CuS<sub>4</sub> + 4Zn <s> ></s> 4ZnS +Cu
might be useful in turquoise-blue stars when binded.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
If there's any newer thread, please merge it with this post. (can't believe last posts on this interesting subject are dated 2003  ) )
I was testing effects of some impurities on zinc sulfide and I found out that silver and strontium (as nitrates) and sodium (as chloride) don't do
anything in particular.
My plan was to mix the powder in silicone grease and press it between two microscope slide covers, to make a scintilator.
Copper is the winner, and I used a grinded mixture of chloride, sulfate and the metallic powder itself, in minute quantities.
I've mixed zinc (reagent grade) and sulfur (technical grade) with the copper dopants, put it in a steel can, covered it with an asbestos mat and
heated until a vigorous reaction has occured, which made the whole can glow intensively orange. The asbestos mat was to avoid the oxidation of the
sulfide, and to catch the product as much as possible. Brilliant flame and smoke oozed beneath the mat and left some interesting bits for analysis.
What I've noticed is that not all of the product in the crucible glows with equal intensity under blacklight tube.
The most striking glow was exactly at the places where that flame and smoke were oozing out, depositing almost white flakes.
Actually, that white smoke is the key. Exactly that aerosol is the precious intensively phosphorescent matter, and it is usually
wasted as it billows in the atmosphere.
Residues in the crucible are nothing compared to the stuff that is lost. That's probably the reason why ZnS is neglected by the amateurs - they don't
catch the best part, and let it out. 
And perhaps additional reaction with oxygen is needed, too.

You can see that the best glowing is around the edge.
The asbestos mat captured at least some of the powder that was flowing outside, and as you can see in this photo, the glow is striking.
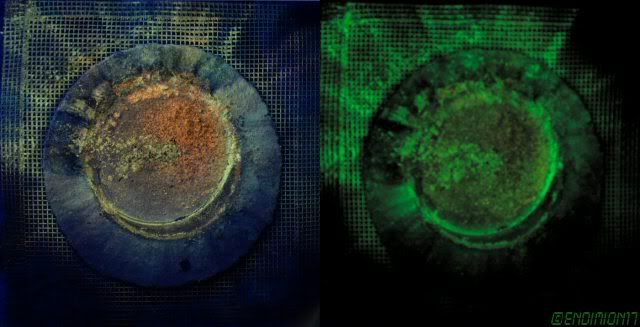
I've tweaked the image to appear as I see it with my own eyes after the dust is charged by blacklight tube.
It's obvious that only the lightest particles which flowed outside glow the most.
The product is rather heterogenous. Maybe it has something to do with particle sizes.
Tomorrow I'm capturing the smoke in a long glass tube, calcinating the residues from the crucible in the absence of air and in air, and I'm grinding
the rest to check for particle size effect.
So stay tuned...
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
I've did it. Though it wasn't very sucessful. 
Grinding did nothing. It's useless.
Calcinating, whether it was done openly or in an tube reduced the phosphorescent properties. I've let it stay orange-hot and red hot for 5-10 minutes.
Still hot compound won't glow at all, and when it cools down, its glow is a lot weaker compared to before.
I've tried to catch the smoke with a 1.5 meter long glass tube (somewhat similar to a very, very crude cromatography), and found out that only parts
of it low appreciately, as you can see in this photo.
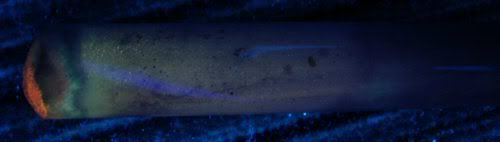
The colors are a bit messed up because the tube attenuates UV rays a bit, but the parts that glow are obvious.
It's puzzling. My current opinion is that ZnS doped with copper will glow well only if it's "forged in hell", but afterwards cooled at a fast rate.
And traces of oxygen might help, too.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
The biggest problem with inorganic scintillators is that they need to be extremely pure in order to work well. I've been considering making some zinc
sulfide myself. The method I wanted to employ involved the use of hydrogen sulfide. Bubbling H2S through a clean solution of a zinc salt will yield
very pure zinc sulfide. Unfortunately, H2S is a notorious poison, so if you do this, be careful.
[Edited on 8-16-2011 by White Yeti]
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I tried making zinc sulfide with zinc acetate and hydrogen sulfide, complete and utter failure. I did add a little copper acetate to the solution in
attempt to "dope" it, so that wasn't the problem. I got a precipitate, but it didn't glow at all. I could try it again, I have a little aluminium
sulfide left over.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
annaandherdad
Hazard to Others
  
Posts: 388
Registered: 17-9-2011
Member Is Online
Mood: No Mood
|
|
When I was a teenager (early 1960's) I had a zinc sulfide scintillator as a part of a lab kit on radioactivity. It was a screen inside a plastic
container with a lens you could look through.
In those same years I took a course in qualitative analysis at the local college. We routinely made hydrogen sulfide and bubbled it through solutions
(Pb etc) to get precipitates to identify compounds. I remember the teacher saying that hydrogen sulfide was poisonous, but it smelled so bad that no
one had ever been known to be poisoned by it. In any case, we didn't worry about it during the lab, and, apart from that remark, neither did the
teacher. The lab smelled bad, but no one died.
Amazing how times have changed.
Any other SF Bay chemists?
|
|
|
CrEaTiVePyroScience
Hazard to Self
 
Posts: 71
Registered: 14-4-2012
Location: Belguim
Member Is Offline
Mood: Explosive
|
|
http://www.youtube.com/watch?v=5HtNCdz8X3g
=
ZnS "synthesis" + reaction
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by annaandherdad  | When I was a teenager (early 1960's) I had a zinc sulfide scintillator as a part of a lab kit on radioactivity. It was a screen inside a plastic
container with a lens you could look through.
In those same years I took a course in qualitative analysis at the local college. We routinely made hydrogen sulfide and bubbled it through solutions
(Pb etc) to get precipitates to identify compounds. I remember the teacher saying that hydrogen sulfide was poisonous, but it smelled so bad that no
one had ever been known to be poisoned by it. In any case, we didn't worry about it during the lab, and, apart from that remark, neither did the
teacher. The lab smelled bad, but no one died.
Amazing how times have changed. |
Hydrogen sulphide is still being used in teaching except in nanny states. It is harmless in amounts used in analytical chemistry and presents no more
harm than opening a large, smelly bottle of sodium sulphide solution.
Nothing can happen.
I don't understand two things:
1. quotation marks for synthesis
2. saying that zinc and sulphur both react as reductors when they're clearly not. Zinc is oxidized from state 0 to +2, and sulphur is reduced from 0
to -2. Zinc is the reductor, sulphur is the oxidizer. Basic redox example.
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
ZnS is so old school, it's barely worth even the garage chemists attention, unless you happen to be nostalgic about the history of phosphorescence
over the years,
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I respect your opinion, but how many simple alternatives are there to zinc sulfide? Once you start using rare earths and furnaces, this modest venture
turns into an onerous project for the average chemist.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Squall181
Harmless

Posts: 46
Registered: 21-2-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  | | I tried making zinc sulfide with zinc acetate and hydrogen sulfide, complete and utter failure. I did add a little copper acetate to the solution in
attempt to "dope" it, so that wasn't the problem. I got a precipitate, but it didn't glow at all. I could try it again, I have a little aluminium
sulfide left over. |
Did you fire the powder after precipitation? When you percipitate zinc sulfide from solution it is in its amorphous form. You have to heat it up to
get it to crystallize and become phosphorescent.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Here is a detailed reference (http://zinc.atomistry.com/zinc_sulphide.html ) on ZnS, but I will let the readers, who have worked with the salt, discern the level of quality. To
quote:
"methods of obtaining crystalline zinc sulphide (wurtzite) are -
a. Heating zinc oxide or silicate in sulphur vapour at a red heat.
b. Interaction between zinc vapour and hydrogen sulphide.
c. Melting together zinc sulphate, barium sulphide, and calcium fluoride.
d. Action of zinc chloride vapour, diluted with carbon dioxide, on some metallic sulphides, especially tin sulphide.
Crystals of blende are said to be obtained by heating zinc sulphide in a solution of hydrogen sulphide under pressure, by subjecting zinc chloride
vapour to the action of hydrogen sulphide, and by the action of carbon disulphide on zinc oxide at a white heat.
White amorphous zinc sulphide is precipitated by passing hydrogen sulphide through solutions of zinc salts. Since it is less soluble than the oxide or
carbonate, suspensions of these in water are converted into sulphide by hydrogen sulphide. Zinc sulphide is perceptibly soluble in sodium hydrogen
sulphide, freely in mineral acids, and somewhat soluble in ammonium chloride on boiling.
The precipitation of zinc sulphide by passing hydrogen sulphide through solutions of its salts is not usually quantitative: this is usually explained
by the reversibility of the reaction
ZnCl2+H2S ⇔ ZnS+2HCl.
According to Glixelli, the reaction
ZnSO4+H2S = ZnS+H2SO4 [corrected]
is not reversible, partial precipitation arising from false equilibria, and prolonged passage of hydrogen sulphide, at ordinary temperature, through
¼ and ½ molar solutions of zinc sulphate, precipitates the zinc [meaning ZnS?] completely.
When zinc sulphide is precipitated in media in which it is slightly soluble, as in the presence of weak acids, it is either crystalline or becomes so
on standing. According to Villiers, it is soluble in sodium hydrogen sulphide at the moment of precipitation, and passes from this etat protomorphique
into a crystalline insoluble form with a rapidity which varies with conditions. According to Glixelli, the β-sulphide precipitated from alkaline
solutions is forty-six times as soluble as the a-sulphide precipitated from acid solutions.
When zinc sulphide is first precipitated in media in which it is very insoluble it readily goes into colloidal solution. A colloidal solution has been
prepared by precipitation with hydrogen sulphide in ammoniacal or neutral solution and washing with hydrogen sulphide water, and by passing hydrogen
sulphide into an aqueous suspension of zinc oxide. The addition of glycerine, or other substances increasing the viscosity of the solution,
facilitates the formation of these colloidal solutions, which are milky by reflected and orange by transmitted light.
Seligmann obtains a readily filterable zinc sulphide by heating a strongly ammoniacal solution, containing 0.5 grm. zinc in 200 c.c., to 60° or 80°
C. and adding a slight excess of ammonium sulphide.
Zinc sulphide is tinged light brown or grey by exposure to light or heating to 60°-70° C. This behaviour, which may be due to polymerisation, is
promoted by various substances and inhibited by others, and is important for the use of zinc sulphide as a pigment, whether alone or, mixed with
finely divided barium sulphate, in lithophone.
The ordinary sulphide, natural or artificial, phosphoresces after exposure to light. This phosphorescence is affected by traces of other metals, but
the data seem to be somewhat contradictory. A similar phosphorescence is also stimulated by exposing the sulphide to the action of ozone. Apparently
there is no phosphorescence in ordinary precipitated zinc sulphide, but if it is heated for about one and a half hours at 650°-900° C. it will
phosphoresce under light, Becquerel rays, X-rays, cathode rays, and radioactive emanations. The favouring conditions for phosphorescent behaviour seem
to be semi-crystalline condition and the presence of chlorine ions. The latter may strain the particles of zinc sulphide by coating them with zinc
chloride. The luminescence of crystalline zinc sulphide under X-rays is increased by the presence of 1.30 per cent, of cadmium sulphide in solid
solution. "
[Edited on 18-10-2012 by AJKOER]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Impurities even at extremely low levels create traps which wreck the desired result. Also important is particle size and improper mixing. One point
missed by most is attention to atmosphere. Plug the ends of your tube furnace with a material such as Kaowool. Insert a smaller quartz tube to run the
proper gas in while at high temperature. For ZNS/Ag or Cu you need O2. However for Lanthanide doped Aluminates O2 must be avoided, you need H2 or a
reducing atmosphere. O2 will poison the long persistence phosphors. During the times in the furnace you should not be reducing, a slow Argon flow is a
must. Otherwise the glow will drop from a dozen or more hours to mere seconds. I know as my powders which have a persistence of around 24 hours took a
long time to perfect. As an example I tried using a file and sawing (not both at once) to get flakes from some of my dopant metals which are extremely
hard to work, for use in my doping. Failure always. Turned out even PPM or likely PPB traces of iron is so poisonous to persistence that failure is
guaranteed. Fe traps are detrimental. In the end with home experimenter techniques the only simple method is using a fiber cutting wheel on a Dremel
tool to get a small chunk of the desired metal. Then dissolving this to use in making a salt of the metal, filtering and recrystallization for purity.
The salt which works best is a nitrate by the way.
The next failure by experimenters is failure to use the proper flux while processing in the kiln. There are many useful patents to study before you
experiment, a great money and time saver. A quick and dirty way to achieve decent persistence is experimenting with Calcium Aluminates, Eu doping,
using a blowtorch (only using the proper reducing flame area) in a boat of Al2O3. This method fails in ZnS Ag scintillation material experiments. You
just cannot control the atmosphere well enough. Sometimes a small area will work after crushing. Typically this portion of material was inside a
larger piece of the material and thus more protected from the air.
glow patents
--------------------
2675331
2780731
2792447
2859367
2863084
2881353
2892096
2915661
-------------------------------------
3291747
3294699
3306768
3420861
3441512
3449259
3458451
3497458
3503006
3564322
3574130
3577350
3623994
3657140
3669897
3725811
3728594
-----------------------
4032471
4096088
4097776
4110660
4150321
4153572
4161457
4216408
4249108
4263164
4374037
4382207
4423349
4441049
4416933
4442377
4497785
4508769
4529410
4585673
4590405
4631144
4690832
4691140
4720436
4727004
4733126
4734618
4751427
4783596
4795588
4827187
4837481
4847533
4840747
4855189
4857228
4990371
4999219
------------------------
5015452
5043097
5045289
5057363
5080928
5112759
5141671
5150006
5151629
5156885
5216134
5220243
5244750
5273681
5350971
5358734
5376303
5405709
5405710
5411398
5418062
5424006
5431851
5432014
5466392
5484922
5516577
5545386
5593782
5611959
5637258
5667724
5672200
5714835
5879586
5932968
---------------------------------
6045721
6096243
6117362
6180029
6187225
6210777
6290875
6310118
6312835
--------------------------
electroluminescent pats
---------------------
2421207
2544507
3894164
3898174
3984584
4348299
4374037
4389973
4416933
4442377
4594528
6,215,243 Radioactive cathode emitter for use in field emission display devices
6,143,201
IR phosphors
--------------------------------
2979467
3066222
3610932
3859527
3959658
4064066
4236078
4705952
---------------------------------------------------------
aluminum hydroxide
------------------------------
5492542
6,887,454 Process for the production of aluminum hydroxide
4234559
4340579
4530699
4574074
5130113
5158577
6,887,454 Process for the production of aluminum hydroxide
6,162,413 Alpha-alumina and method for producing same
6,106,800 Method for producing alpha-alumina powder
5,916,536 Production of calcined alumina with a crystallite size which can be regulated with a narrow dispersion
5,445,808 Process for preparing ultra-white alumina trihydrate
5,225,229 Aluminum hydroxide production
5,149,520 Small sized alpha alumina particles and platelets
-------------------------------------------------
4,606,846 Mixed rare earth metal, divalent transition metal, aluminum oxide
3,931,591 Q-switching device for glass lasers
3,931,590 Q-Switching device for glass lasers
4,115,312 X-ray fluorescent luminescent cadmium tungstate compositions
6,407,020
6699598 Laminar body having phosphorescent properties, process for producing it and its use
6,654,079 Liquid crystal color display screen comprising a phosphor layer
6,528,186 Stratified composite with phosphorescent properties, method for the production and the use thereof
6,398,970 Device for disinfecting water comprising a UV-C gas discharge lamp
6,190,577 Indium-substituted aluminate phosphor and method for making the same
5,985,174 Fluorescent material used in an active dynamic liquid crystal display device and method for manufacturing
the same
5,575,050 Method of assembling electronic component
5,402,036 Low pressure mercury vapor discharge lamp having double layers
5,105,121 Lanthanum cerium aluminate phosphor and an electrical discharge device containing the same
5,015,497 Cathode for electron tube and manufacturing method thereof
4,837,481 Cerium and terbium activated luminescent material and mercury vapor discharge lamp containing the same
4,800,319 Low-pressure mercury vapor discharge lamp
5,065,023 Solid state high resolution photography and imaging using electron trapping materials
4,733,126 Phosphor and fluorescent lamp using the same
4,518,985 Projection type green cathode ray tube, method for manufacturing phosphor screen for the same, and projection video device using the same
4,382,207 Luminescent material and discharge lamp containing the same
4,983,834 Large area particle detector system
4,940,603 Thin film inorganic scintillator and method of making same
4,915,982 Method of making thin film photoluminescent articles
4,879,186 Photoluminescent materials for outputting reddish-orange light and a process for making the same
4,855,603 Photoluminescent materials for radiography
4,842,960 High efficiency photoluminescent material for optical upconversion
4,839,092 Photoluminescent materials for outputting orange light
4,830,875 Photoluminescent materials and associated process and infrared sensing device
4,822,520 Photoluminescent materials for outputting blue-green light
4,818,434 Thermoluminescent material including fusible salt
4,812,660 Photoluminescent materials for outputting yellow-green light
4,812,659 Infrared sensing device outputting blue-green light
4,806,772 Infrared sensing device outputting orange light and a process for making the same
4,755,324 Thermoluminescent material
4,348,299 Method for preparing inorganic sulfides
3,977,991 Manganese-and-magnesium-activated strontium sulfide phosphors
6,684,557 Process for making an aquatic lure phosphorescent and charging same with an ultraviolet light
6,346,326 Coated moisture impervious red phosphors
4,374,037 Method for preparing divalent-europium-activated calcium sulfide phosphors
5,006,366 Photoluminescent material for outputting orange light with reduced phosphorescence after charging and a process for making same
4,992,302 Process for making photoluminescent materials
4,857,228 Phosphors and methods of preparing the same
4,855,603 Photoluminescent materials for radiography
4,842,960 High efficiency photoluminescent material for optical upconversion
4,839,092 Photoluminescent materials for outputting orange light
4,830,875 Photoluminescent materials and associated process and infrared sensing device
4,822,520 Photoluminescent materials for outputting blue-green light
4,812,660 Photoluminescent materials for outputting yellow-green light
4,806,772 Infrared sensing device outputting orange light and a process for making the same
4,436,646 Green-emitting phosphor for cathode-ray tube
4,075,495 X-ray luminescent screen
4,057,507 Europium and samarium activated rare earth oxysulfide phosphor
3,950,668 Cathode ray tube containing silicon sensitized rare earth oxysulfide phosphors
6,346,326 Coated moisture impervious red phosphors
4,983,834 Large area particle detector system
4,864,536 Optical memory system and method of using the same
4,857,228 Phosphors and methods of preparing the same
4,855,603 Photoluminescent materials for radiography
4,842,960 High efficiency photoluminescent material for optical upconversion
4,839,092 Photoluminescent materials for outputting orange light
4,830,875 Photoluminescent materials and associated process and infrared sensing device
4,822,520 Photoluminescent materials for outputting blue-green light
4,818,434 Thermoluminescent material including fusible salt
4,812,660 Photoluminescent materials for outputting yellow-green light
4,806,772 Infrared sensing device outputting orange light and a process for making the same
4,755,324 Thermoluminescent material
4,057,507 Europium and samarium activated rare earth oxysulfide phosphor
6,081,069 Phosphor, cathode-ray tube, fluorescent lamp and radiation intensifying screen
5,814,932 Phosphor, cathode-ray tube, fluorescent lamp and radiation intensifying screen
5,808,409 Phosphor, cathode-ray tube, fluorescent lamp and radiation intensifying screen
5,644,193 Phosphor, cathode-ray tube, fluorescent lamp and radiation intensifying screen
4,526,873 Transparent, mullite glass-ceramics containing ZnO and method
4,328,299 Polychromatic glasses and method
4,167,417 Fluorescent inorganic pigment
4,102,805 Cathodoluminescent and photoluminescent glasses
3,962,117 Cathodoluminescent glasses activated by manganese
3,935,119 Luminescent device, process, composition, and article
6,684,557 Process for making an aquatic lure phosphorescent and charging same with an ultraviolet lig
6,039,894 Production of substantially monodisperse phosphor particles
5,958,591 Electroluminescent phosphor particles encapsulated with an aluminum oxide based multiple oxide coating
5,917,279 Intermediate layer in electroluminescent arrangements containing finely divided inorganic particles
5,908,698 Encapsulated electroluminescent phosphor and method for making same
5,619,098 Phosphor and fluorescent display device
5,593,782 Encapsulated electroluminescent phosphor and method for making same
5,439,705 Encapsulated electroluminescent phosphor and method for making same
5,431,851 Composite electroluminescent phosphor
5,418,062 Encapsulated electroluminescent phosphor particles
5,366,834 Method of manufacturing a cathode ray tube phosphor screen
5,309,071 Zinc sulfide electroluminescent phosphor particles and electroluminescent lamp made therefrom
5,294,368 Method of making a composite electroluminescent phosphor
5,156,885 Method for encapsulating electroluminescent phosphor particles
5,094,185 Electroluminescent lamps and phosphors
4,961,956 Electroluminescent lamps and phosphors
4,855,189 Electroluminescent lamps and phosphors
4,181,753 Process for the production of electroluminescent powders for display panels and coating the powders with zinc phosphate
4,097,776 Coated electroluminescent phosphors
4,024,298 Method of providing storage dielectric of phosphor particles coated with secondary emissive material
4,021,588 Method for preparing filter-coated phosphor particles
5,105,121 Lanthanum cerium aluminate phosphor and an electrical discharge device containing the same
4,840,747 Method for producing a terbium activated cerium magnesium aluminate phosphor
4,757,233 Efficient UV-emitting phosphors based on cerium-activated calcium pyrophosphate and lamps containing the same
4,441,049 Luminescent material and discharge lamp containing the same
4,246,630 Ultraviolet emitting Ce alkaline earth aluminate lamp phosphors and lamps utilizing same
4,153,572 Ultraviolet emitting CeYMg aluminate fluorescent lamp phosphor for psoriasis treatment
4,088,922 Cerium magnesium aluminate luminescent compositions, and lamps utilizing same
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
Squall181
Harmless

Posts: 46
Registered: 21-2-2011
Member Is Offline
Mood: No Mood
|
|
This is a picture of Zinc Sulfide Phosphorescence. I am currently working on a instructional video of how to make this. Strong phosphorescence lasts
for over a minute and the material weakly phosphorescence for hours.
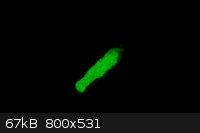
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Quote: Originally posted by Squall181  |
Did you fire the powder after precipitation? When you percipitate zinc sulfide from solution it is in its amorphous form. You have to heat it up to
get it to crystallize and become phosphorescent. |
I will admit, I didn't crystallise it. I don't have the equipment to get up to 1200C. Although I could borrow a bunsen burner...
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Squall181
Harmless

Posts: 46
Registered: 21-2-2011
Member Is Offline
Mood: No Mood
|
|
Here are the links to my first two videos on the synthesis. I still need to edit and upload the last one, which should be soon.
This one is just really and intro and it sounds scripted because it is.
http://youtu.be/aBTTNbssp24
This one shows the synthesis and purification of Zinc Sulfate a precursor for Zinc Sulfide
http://youtu.be/ao4T27UUtq0
The next video will cover the precipitation of Zinc Sulfide from the Zinc Sulfate solution. It will also cover the doping and firing procedure.
[Edited on 12-12-2012 by Squall181]
|
|
|
|