dobeid
Harmless

Posts: 7
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
Questions about how to boil under reflux
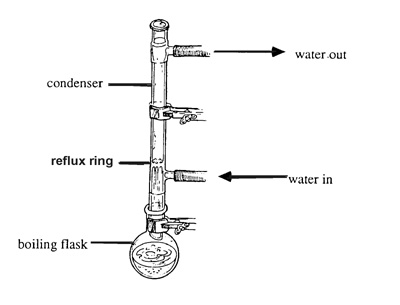
This is my first experiment where I need to 'boil under reflux'. Could someone please explain the exact proper procedure for 'boiling under reflux". I
am specifically looking for clarification on what I am suppose to do or not do with the openings of the coiled condenser. When boiling to reflux, is
the top of the condenser sealed with a glass plug or is it left open/unplugged? Also, is the water inlet and outlets sealed or left open?
Thanks very much.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Basically you are boiling something but instead of lettig it evaporte away unreacted it is condensed and returned to the boiling flask to react again,
say for example the oxidation of ethanol to acetic acid with acidified K2CrO7, if you boil it off immeidetly you will get a large amount of
acetaldehyde however if you reflux it nearly all the ethanol will be oxidised.
Normally the top of the condenser is left open. By water inlet and outlet i imagine you mean of the condenser. The answer is it depends. If you are
using a solvent that boils at 145degrees C then a air condenser would be adequite to condense the vapours. If you are using something like ethanol you
should use a water condenser (preferably a coil conenser)
p.s. shouldnt this be in the short questions thread?
[Edited on 19-9-2008 by Picric-A]
|
|
|
dobeid
Harmless

Posts: 7
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
THANKS!
thanks very much. I'm new to this site so I'll make sure I put any other questions in the short list area. Again, thanks for clarifying.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Don't put a stopper in the top of the condenser or you will be heating a sealed system.
At some point the stopper will pop out!
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
If you are trying to heat something under the cover of a as, eg, argon, put the stopper on very lightly so if there is sufficiant preassure build up
it wont blow off but will leak out,
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Or you could use a bubbler which is the proper way and much safer.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Isnt it a bit risky using a bubbler? as you heat it the gas will expand out but when it cools it will pull the water in.... Lethal when you are
preparing grinards,
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Not if you use a proper bubbler and something other than water (mineral oil). You could always put in an extra trap if needed in case of suck back.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
| Quote: | Originally posted by bfesser
Not if you use a proper bubbler and something other than water (mineral oil). You could always put in an extra trap if needed in case of suck back.
|
Pray tell mystical sir how this fabled 'proper' bubbler differs from a normal bubbler, is it just the presence of a glass non-return valve in your
line?
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
There's several designs...
If the pressure in the apparatus is reduced, the mineral oil simply gets sucked into the upper chamber of the bubbler:

In this variation, the tubing has bulbs which prevent the low liquid volume from being sucked all the way through (it's a little hard to see):

This one's easy enough to make:

And I've seen single piece glass ones of similar design, something like this (sorry, after Photoshopping the last one I just got lazy and opened MS
Paint...):

[Edited on 9/19/08 by bfesser]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I like the balloon trick. Put a "T" in the gas line and a balloon on the stem of the "T". Get a little inflation and that maintains positive
pressure on the system. Usually you pull a vacuum couple of times replacing the evacuated atmosphere with your gas. You might get away with
"flushing" if you used Argon but it tends to be wasteful. BTW- get a copy of Organic Chem Lab Survival Manual. The reflux question and many others
were covered there and the book is here in the library.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
benzal
Harmless

Posts: 9
Registered: 21-12-2007
Member Is Offline
Mood: No Mood
|
|
lol, i thought dobeid was asking how to boil and reflux at the same time, listen to picric-A dude
|
|
|
Ozonelabs
Hazard to Others
  
Posts: 120
Registered: 5-4-2008
Member Is Offline
Mood: Oligomerised
|
|
Boiling under inert atmosphere
Instead of a bubbler ozonelabs has found a very good setup for an inert atmosphere reflux.
Ozonelabs adds a quickfit steam inlet tube to a quickfit T-adaptor and runs the inert gas, in this case Nitrogen, into the T-adaptor. This is located
at the top of the condenser.
In this example Ozonelabs is drying Tetrahydrofuran over Sodium with Benzophenone, prior to a distillation.


[Edited on 28-9-2008 by Ozonelabs]
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Nice setup Ozonelabs and good idea!
How do you pull the vaccum needed to flush out the setup with N2?
|
|
|
Ozonelabs
Hazard to Others
  
Posts: 120
Registered: 5-4-2008
Member Is Offline
Mood: Oligomerised
|
|
Hello Picric-A-
Here at Ozonelabs we dont use a vacuum, we just use a vast over pressure of Nitrogen for a few minutes to flush the system.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Thanks, It loooks like your gas inlet is at the top of the condenser,
surly it would be better then to ave your gas inlet joint at the base of the condenser that means flushing the way you do would be more effective?
just a thought... 
|
|
|
Ozonelabs
Hazard to Others
  
Posts: 120
Registered: 5-4-2008
Member Is Offline
Mood: Oligomerised
|
|
Indeed thats the conventional wisdom, however when refluxing with some of the more volatile solvents- Et2O, THF etc. we have found that the upwards
flow of N2 gas actually carries away the solvent vapour without it condensing.
The major issue with this is found when refluxing LiAlH4 in THF and then losing all of the THF!
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Yea i can see that... thanks Ozonelabs, i stand corrected 
|
|
|
Agent MadHatter
Harmless

Posts: 45
Registered: 22-7-2009
Member Is Offline
Mood: No Mood
|
|
New to chemistry, help with Refluxing?
Hello everyone! This is my first post, just joined the forums. I've had...A LOOOOONG time ago, one class in chemistry in highschool. (5 years ago) So
I'm very rusty.
I was reading a post on another forum about refluxing a chloroform solution...but I'm unsure how to do this.
Mainly because I thought refluxing was boiling a solution in a distillation chamber. But I guess I was wrong.
Can someone enlighten me?
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
Behold...the magic of Google http://en.wikipedia.org/wiki/Reflux
[Edited on 23-7-2009 by crazyboy]
|
|
|
Agent MadHatter
Harmless

Posts: 45
Registered: 22-7-2009
Member Is Offline
Mood: No Mood
|
|
I've seen this. But I was thinking more along the lines as a general guideline to refluxing in general.
|
|
|
jwarr
Hazard to Self
 
Posts: 85
Registered: 25-6-2009
Member Is Offline
Mood: No Mood
|
|
youtube has very informative videos on basic ochem lab techniques, including refluxing.
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
It's really easy just put your liquid in a round bottom flask attach a condenser going up run cold water through the condenser and heat the flask.
|
|
|
Nicodem
|
Threads Merged
22-7-2009 at 23:19 |