| Pages:
1
2 |
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Rusting from HCl Bottle
I have been noticing some rusting in my lab originating from my gallon bottle of HCl. I put a piece of aluminium foil over the cap as a sacrificial
layer, although it corrodes rather quickly. Anybody else have solutions?
[Edited on 8-12-18 by Abromination]
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Put it in glass bottles that actually seal.
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
I'm not spending money on a gallon glass bottle with teflon cap...
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I used to have the same problem with everything rusting in my lab. I have multi litre quantities of nitric, sulphuric and hydrochloric to store and
it was eating my lab.
I bolted a large metal cupboard to the concrete floor in a separate small locked shed with an expensive lock to stop things getting stolen and store
all acids in there.
No more problems in the lab since then and I have smaller 500ml duran bottles with good lids I fill from the large containers which I store in the lab
fridge for experiments.
So yeah, just store it elsewhere were it can't damage anything expensive in your lab.
Be good, otherwise be good at it 
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Bulk volatile acids can go in a sealed plastic tub. Cover the bottom of the tub with a generous amount of sodium bicarbonate. Put your bottles in.
Shut the lid. And if you can put the entire unit somewhere reasonably well ventilated, then even better. This won't completely solve the problem but
it will help a lot.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
There aren't that many way to deal with corrosive vapors:
1. Get a better bottle that actually seals (actually even the Duran PTFE red caps leak over time, albeit slowly)
2. Use a sacrificial material to capture the vapors (Sodium bicarbonate, aluminum foil, etc.)
3. Store it where there is nothing to corrode (outside)
Best option is number 1. combined with number 2. If you don't want to spend on number 1. just use a box full of Al foil. It will take a long time to
corrode all of it, and Al foil is much cheaper than replacing your tools and furniture.
|
|
|
monolithic
Hazard to Others
  
Posts: 436
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Don't buy from scientific suppliers, they're scam artists.
Gallon glass bottle: https://www.amazon.com/Home-Brew-Ohio-Polyseal-Capacity/dp/B...
38mm PTFE cap: https://www.usplastic.com/catalog/item.aspx?itemid=23577&...
|
|
|
Mr. Rogers
Hazard to Others
  
Posts: 184
Registered: 30-10-2017
Location: Ammonia Avenue
Member Is Offline
Mood: No Mood
|
|
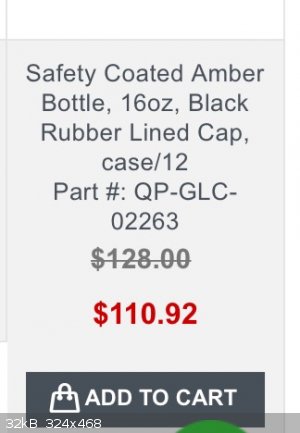
Well, that price is for a case of 12 bottles. That's a poor scam if that's what they're trying to pull.
|
|
|
monolithic
Hazard to Others
  
Posts: 436
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Those are 16 oz bottles in the screenshot. My link is for a 128 oz bottle. If you want 16 oz bottles, they're about $20 for a 12 pack on Amazon.
[Edited on 8-12-2018 by monolithic]
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
My semi-facetious suggestion: If you need a gallon size glass jug, buy a gallon of table wine. Drink the wine. There must be somewhere that you can
get a thin sheet of PTFE.
Cut a liner for the wine jug cap from the PTFE.   
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
They are plastic coated, as mandated by safety codes for acids. That adds to the cost a bit.
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I dilute some of my 36% HCl to 1M for daily use that I keep out on the shelves in a glass bottle,
there are no visible signs of corrosion nearby.
My 36% HCl, which is well above azeotropic, I keep in a hdpe bottle in a pp tub.
The same tub that I keep my 33% ammonia solution in - which seems to leak gas at a similar rate.
Result, no obvious smell of HCl or NH3 but white dust in the tub.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Get a 5 gallon bucket with a lid, either repurposed or from a home improvement store (just a couple bucks). Pour a container of bicarb into the
bottom of the bucket. Sit the bottle of HCl in the bucket, place the lid on top. Do not bother sealing the lid, they can be a pain to remove, just
sit something on top.
|
|
|
I_love_Diethylether
Harmless

Posts: 4
Registered: 12-11-2018
Member Is Offline
|
|
So, what i usually do with corrosive volatile stuff. I take these PTFE toaster bags, cut out a square, tape some PTFE tape around the bottle-plug and
put the foil in between the plug and the opening. In the end i wrap the opening with a generous amount of gladwrap. Worked out for me so far.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
I think the ammonia companion bottle is a better idea than NaHCO3 because each mole of NaHCO3 reacted with acidic fumes makes over 22 liters of CO2!
Depending on ventilation, where (basement more problematic) and how big is ones lab may become deadly significant, especially if there is a visit from
a small pet!
[Edited on 9-12-2018 by AJKOER]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by AJKOER  | I think the ammonia companion bottle is a better idea than NaHCO3 because each mole of NaHCO3 reacted with acidic fumes makes over 22 liters of CO2!
Depending on ventilation, where (basement more problematic) and how big is ones lab may become deadly significant, especially if there is a visit from
a small pet!
[Edited on 9-12-2018 by AJKOER] |
I keep my ammonia in the fridge to minimise losses. It is less easy for me to get than HCl. I suppose I could keep my HCl in there but at significant
loss of space. I carry significantly different volumes of the two reagents.
As for the build up of CO2. Not really a problem. One litre of HCl vapor produces a litre of CO2 by the method I suggested. You could knock this
down by half or to zero by using Na2CO3 or NaOH. But I like what is cheap and available and not hygroscopic. You are not going to gas any critters
with CO2 any more than you currently are with HCl. In my case I am fortunate to have really good ground level ventilation and so no way that gases
can build up anyway.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by AJKOER  | I think the ammonia companion bottle is a better idea than NaHCO3 because each mole of NaHCO3 reacted with acidic fumes makes over 22 liters of CO2!
Depending on ventilation, where (basement more problematic) and how big is ones lab may become deadly significant, especially if there is a visit from
a small pet!
[Edited on 9-12-2018 by AJKOER] |
22 liters of "acidic fumes" produce 22 liters of CO2, better CO2 than ammonia or HCl
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
A slowly leaking gallon jug of acid reacting with a tub of baking soda will NEVER produce dangerous concentrations of CO2 in a room. Change
my mind.
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
The rate of effusion of hydrogen chloride through the bottle and cap would be of an entirely different magnitude than the diffusion of the created
carbon dioxide. If I took a box of bicarbonate, put it in a bucket and poured the entire gallon of acid into the bucket I would not have worry one
about suffocating on the carbon dioxide vapor.
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
I've found bleach bottles seal HCl very well, especially pool shock bottles. The 32% HCl here comes in the same bottles as the pool shock. HDPE with
a HDPE lid, some with a foil lined lid, with a plastic coating on the foil. Put the cap on tight and it should be alright.
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
@RogueRose
That is precisely the bottle & cap type that I keep my 36% HCl in,
the bottle of HCl that needs a bottle of ammonia solution nearby
as judging by the pattern of ammonium chloride dust
HCl gas can diffuse through the wall of the bottle.
(no proof or reference, just an observation)
P.S. 96% H2SO4 in hdpe produces more white dust than 36% HCl on the bottle walls,
I guess because ammonium sulphate is less volatile than ammonium chloride.
96% H2SO4 also discolours over time in a hdpe bottle which is why I transferred mine to glass bottles.
I've not noticed much 'dust' on 69% HNO3 bottles in the same tub
- I've no idea why it is different.
[Edited on 11-12-2018 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
I feel that I should mention it is an HDPE bottle with a HDPE cap, and the acid is azeotropic.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Use empty coke bottles for HCl storage there is no need for glass. the hydrochloric acid bottles suck.the only thing that coke bottles can't hold are
H2SO4.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
It's weird how OTC sulfuric acid drain cleaners are often sold in bottles in bags, but the rows and rows of conc. HCl (potentially rusting the entire
stock of the store) are never in bags.
|
|
|
r0749547
Harmless

Posts: 14
Registered: 21-12-2018
Member Is Offline
|
|
I use HDPE bottles.
you can buy some for about 2 euros/piece in a brewery store.
|
|
|
| Pages:
1
2 |