| Pages:
1
2
3 |
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Preparation of methyl tosylate, safe methylating agent
The most routinely used methylating agents, namely dimethyl sulfate (DMS) and methyl iodide (MeI), present significant health hazard, as both of them
are very toxic.
Several safer substitutes exists, such as triemthylphosphate (TMP), sulfonate esters (RSO3Me), and dimethylcarbonate (DMC).
DMC usually requires harsh conditions to acheive methylation, TMP less so.
I have found commercial TMP to be very efficient in O-methylation of phenols ( Methylation of salicylaldehyde thread ), but have had disappointing results with certain N-methylation. There is very little information on the
use of TMP for N-methylation of aliphatic amines in the litterature, so I decided on trying a new methylating agent, methyl tosylate (TsOMe)
p-toluenesulfonate esters are considered as safe, but efficient alkylating agents, on the same order of reactivity as alkyl iodides.
Theses esters can't be obtained by heating the acid with the desired alcohol, the usually preparation uses the sulfonyl chloride, a stable, white
cristallin solid. Although tosyl chloride hydrolyzes to TsOH and HCl in presence of moisture, this reagent is much less prone to hydrolysis than acyl
chlorides, and can be handled without protective atmosphere with minimal lose. TsCl is a cheap and available reagent, which can be used to form a
variety of tosylates, for alkylation or displacements (halogen or azide swap, etc) purposes.
Preparation of methyl tosylate from tosyl chloride
I followed the OrgSyn procedure for methyl tosylate (see notes), at a 200mmol scale.
Reagents
The tosyl chloride, which is quite old, was used without any purification. It surely contained some TsOH. It was weighed and crushed to a fluid powder
inside a home-made drybox, but this isn't mandatory, just avoids caking up and increases the lifespan of the reagent.
Technical methanol, not dried, and commercial 30% NaOH solution were used.
Procedure
In a 250mL 4-neck RBF, equipped with a condenser, a addition funnel, a thermometer and magnetic stirring, 50mL of technical methanol were charged (
~1.2 mol, 6eq.). The flask was cooled in a cold water bath to 10°C.

38.13g (200 mmol) of TsCl were weighed into a dried pyrex container inside a dry box, and the caked-up lumps where broken down to a fine, fluid
powder. The sulfonyl chloride was then added in portions over 5min to the stirred methanol, no heating occured. Stirring was set to obtain a well
stirred suspesnion but avoid any splashing.

20.0mL of 30% NaOh solution (200mmol) were charged into the addtion funnel, and added dropwise (1dp/5sec)to the stirred suspension, keeping the temp
between 20 and 25°C by occasionally adding ice to the water bath. There was a slight exothermic reaction.

As the addition continued, the reaction mixture became more viscous, and the white solid seemed to get thinner. When stirring was stopped, an oily
layer formed at the bottom of the flask, along with the white solid.

The addition took about an hour, and the temp never exceeded 30°C. Some white solid remained unreacted (surely TsOH originally present in the
reagent), and pH was slightly above 7.
the reaction mixture was left to stir for an hour at 15°C, then left to decant for another hour at the same temp.
50mL of water containing ~15g of NaCl was then added, causing immediate milkyness, and the colorless oil that sank to the bottom seperated. The
aqueous was extarcted with 4x15 mL toluene. The extracts were washed with 50mL 5% K2CO3, 50mL water, and 50mL brine. the slightly milky solution was
dried over K2CO3 for 30min, and the solvent removed under reduced pressure (at ~50°C) using a short vigreux, max vacuum applied for 15min to remove
as much toluene as possible.


The viscous, pleasant-smelling ester weighed 32.77g (176 mmol, 87.99 % yield), and must contain some traces of toluene as it didn't
solidify upon cooling to under 30°C.

Comments
This preparation is very easy, requires very little equipment and material, and can be done in a few hours. A workup avoiding the use of toluene could
be usefull, I might try drowning the reaction medium in ice water next time, and filter out the solid.
I prefered on keeping the crude product, and using it pretty quickly, as distn requires tough vacuum (<10mmHg) to avoid decomposition. Apparently,
the crude ester decomposes on standing after a few weeks.
High yeilds of this ester can be obtained, so it seems tosyl chloride is a very good alternative to dimethylsulfate or MeI, both health-wise and
financially, if the chemist is willing to spend a few hours preparing the needed ester. Ther eaction can easily be performed on a large scale,a s
suggested by the OrgSyn procedure, and the controlable exothermic reaction.
More accesable methods, not involving the sulfonyl chloride exist, like refluxing the anhydrous acid with excess trimethyl orthoformate, but high
excess of this ester are required.
The acid can be recycled to the sulfonyl chloride with S2Cl2, but it's a dirty reaction, yielding a impur product. Otherwise, SOCl2, PCl5, etc are
needed.
A better approach, for those that can't aquires TsCl, would be to prepare aliphatic sulfonyl chlorides by reacting alkyl halides and thiourea or
thiosulfates, followed by aqueous chlorination of the intermediate adducts, and reacting these sulfonyl chlorides with the chosen alcohol.
Ullman (SM member) had the great idea of forming ethylsulfonyl chloride from the easily made ethyl halide and sodium thiosulfate, both very common
products, and then reacting that with methanol, ethanol, etc.
Unfortuanly, aromatic sulfonyl chlorides cannot be prepared by this method.
I hope this post can sparkle some interest in these compounds and get people to try their preparation. Several references regarding the chlorination
of isothioureas or Bunte salts (alkylthiosulfates) to sulfonyl chlorides are availble in the ref. forum.
[EDIT: Moved to Organic Chemistry]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
When you talk about TMP don't you mean trimethyl phosphite ((MeO)3P) rather than trimethyl phosphate ((MeO)3P=O) ?
The phosphite ester is a well known methylating reagent.
I do not recall the phosphate ester as an alkylating agent.
As for ethyl tosylate, it would be interesting to see if it can be substituted for diethyl sulfate in the N-alkylation of diphenylurea assisted by
Et3BuN+Cl-. This is the non-phosgene route to enthyl centralite.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
No, no, trimethyl phosphate, (CH3O)3PO. Indeed, the phosphites are also alkylating agents, but also act as nucleophiles IIRC.
These phosphate esters are less reactive than sulfate etsers, but their high bp ables higher reaction temps. At 50-60°C in DMF, TMP was as effective
as MeI for O-alkylation of salicylaldehyde. There is one OrgSyn procedure employing TMP for dimethylation of certain anilines, and a article on the
same subject (recently requested in the ref forum); apart from that, nothing else I could locate on N-alkylation.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
google n-alklylation and cesium you'll like it
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
you use an akly halide and a primary amine in dmf along with mol. seives and Cs(OH)II.H2O
the cesium base promotes mono alkylation over the susequent reactions, yeilds are in the 90's
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Indeed, I was well aware of this route but I have no cesium hydroxide and my supplier is closed until september, aswell as the lab, so I have to do
with what I have at hand...
Have you tried this yourself? It would be a good alternative if this is first-hand yields. The HCHO/Zn procedure seemed very promising at first, but
happened to be shit..
BTW, I think this discussion would be better placed in the monomethylation thread rather than here.
In any case, TsOME is much easier to handle than MeI or DMS, but does have an inconvenient: the form tosylate salt precipitates in large amounts as a
very voluminous solid, making stirring very difficult depending on the solvent used (gel-like paste in THF, but crisatllin flakes in toluene), so
larger amounts of solvents are required. I haevn't tried DMF yet, it's possible the salt is somewhat soluble in that solvent.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Thank you so much Klute for such a brillant and thorough report. (J'apprécie énormément ton travail !)
Could you please, if possible, link me to a study where methyl tosylate has been used as a phenolic methylating agent ?
Should one just substitute methyl iodide to TsOME by adjusting the ratios and thats it ?
Many thanks in advance !
|
|
|
grind
Hazard to Others
  
Posts: 120
Registered: 13-1-2007
Member Is Offline
Mood: No Mood
|
|
Tosylate esters are strong cancerogens, like most alkylating agents. They may have preparative advantages, but are not safer to your health than
dimethyl sulfate or MeI.
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
| Quote: | Originally posted by grind
Tosylate esters are strong cancerogens, like most alkylating agents. They may have preparative advantages, but are not safer to your health than
dimethyl sulfate or MeI. |
Life is a deadly disease.
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Klute, I dont know if you were aware of the following procedure but I will post it for information purposes. It reports that sulfonic acids can be
smoothly converted to their methyl and ethyl esters by reaction with trimethyl and triethyl orthoformate, respectively.
This could prove to be handy if one only has p-toluenesulfonic acid and not the chloride derivative.
A. A. Padmapriya, G. Just and N.G. Lewis
Synthetic Communications 15(12), 1057-1062 (1985)
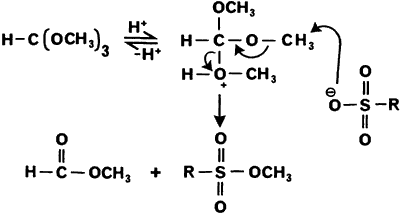
Experimental
General Procedure
A solution of the sulfonic acid (200 mg) in trimethyl (or triethyl) orthoformate (2 mL) was either allowed to stir at room temperature for 14 h., or
heated to ref lux for 30 min., under an atmosphere of nitrogen. The excess orthoformate was then removed under vacuum (0.5 mmHg) to give the
corresponding methyl (or ethyl) ester. Where the solubility of the substrate was poor, the reagent was mixed with an equal volume of methanol,
following which slow distillation over 1 h. gave the required ester in high yield.
****Update****** Sorry, I just read in your post that you already knew about it. I
should be more careful !!!*****
[Edited on 31-12-2008 by Quantum_Dom]
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
| Quote: |
A. A. Padmapriya, G. Just and N.G. Lewis Synthetic Communications 15(12), 1057-1062 (1985) |
For those who want it. 
sparky (~_~)
Attachment: estrsulfo.pdf (136kB)
This file has been downloaded 2300 times
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Very nice job spark 
[Edited on 31-12-2008 by Quantum_Dom]
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
Hello Klute, could yo post a procedure for making p-toluenesulfonyl chloride from p-toluenesulfonic acid and disulfur dichloride for those that cannot
obtain tosyl chloride? I have been wanting some tosyl chloride for some time, I have plently of p-toluene sulfonic acid and disulfur dichloride. It
would be interesting to try to optimize the tosyl chloride synthesis you mention and find a suitabl solvent to remove the tosyl chloride from
impurities introduced by the synthesis.
Amateur NMR spectroscopist
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Hi benzylChloride,
I will have to search the procedure again, I didn't keep any info on it at the time.. Give me a day or two..
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Thanks klute, I appreciate this too.
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
nabh4 and formalin read up
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
benzylchloride: Up to date I have still not found the patent that mentionned the sulfonic acid + S2Cl2 to form sulfonyl chloride.. Actually it was a
patent describing more the purification of sulfonyl chlorides obtained by such method rather than the process itself, but it contained references, and
the simple fact that a patent was filed specificly for products obtained via this method means it sufficiently used in the industry. I have spent
several hours (!!) on many patent search engines with keywords like sulfonyl chloride purification sulfur dichloride, etc etc but have still not
located it.. At the time I just read through it after random searching on sulfonyl chloride, and kept the reaction in a small mind box as a valid
preparation without noting any info.. Useless now..
Jon, NaBH4 and formol gives mainly dimethylated amines, or statistical repartition of mono and dimethylated when formol is used in slightly less than
molar proportions. Found it out in the lab.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Klute  | | benzylchloride: Up to date I have still not found the patent that mentionned the sulfonic acid + S2Cl2 to form sulfonyl chloride..
|
Maybe this one: DE499052?
The only other methods that could be suitable for amateurs are:
- heating PhCCl3 and TsOH at ~150°C to give PhCOCl and TsCl (CN101070295);
- treating TsOH with P4O10/KCl/n-Bu4NCl in MeCN (Bulletin of the Chemical Society of Japan, 56 (1983) 3813-3817; this is just a minor example, because the paper otherwise focuses on the
reduction of arylsulfonic acids to diaryldisulfides with HI);
If you have an autoclave (for up to 10 bar at least!) you can try heating a solution of TsOH*H2O in 1,1,2-trichloroethene at 120-150°C. By theory
this should give a mixture of TsCl, ClCH2COOH and ClCH2COCl. 1,1,2-trichloroethene (aka trichloroethylene) is a widely available solvent used as
degreaser, a thinner to clean brushes, and for dry cleaning.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
zLo0nGz
Harmless

Posts: 3
Registered: 8-8-2010
Member Is Offline
Mood: No Mood
|
|
Dear Klute,
Can your method be used to prepare Isopropyl p-toluene sulfonate (Isopropyl tosylate) from p-toluene sulfonic acid and isopropyl alcohol (IPA)? Do you
have any suggestion on how can this be performed? I have tried refluxing both chemicals with excess of IPA, temperature of 100-110oC, for 24 hours.
Distill of the excess IPA and got a dark greenish semi-solid. Do you have any suggestion to purify this dark green semi-solid?
Thanks in advance.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zLo0nGz  | | Can your method be used to prepare Isopropyl p-toluene sulfonate (Isopropyl tosylate) from p-toluene sulfonic acid and isopropyl alcohol (IPA)? Do you
have any suggestion on how can this be performed? I have tried refluxing both chemicals with excess of IPA, temperature of 100-110oC, for 24 hours.
|
How is refluxing of tosylic acid in isopropanol supposed to give isopropyl tosylate? That is not only impossible, but it is also not what Klute
describes here. I think you misinterpreted something.
|
|
|
zLo0nGz
Harmless

Posts: 3
Registered: 8-8-2010
Member Is Offline
Mood: No Mood
|
|
Dear Nicodem,
Klute was describing about making methyl tosylate...in my case, isopropyl tosylate...now I have looked up in the internet...the only way that I could
make isopropyl tosylate is by reacting tosyl chloride with IPA...however reaction of tosyl chloride with IPA gives HCl as by-product, a little risky
eh...thats why I'm looking for a safer alternative to making isopropyl tosylate, the only way that I can think of is by reacting tosylic acid with
IPA...or is there any other suggestions I could try?
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
HCl shoud be very easy to remove from you final product.
And if you dont want to use tosyl chloride there are other alternatives. Here is an article you may like, but I cant view the whole thing and I dont
want to pay $30.00 to. http://pubs.acs.org/doi/pdf/10.1021/jo01185a005 . Maybe someone else has it, you could ask in references.
|
|
|
Formula409
Hazard to Others
  
Posts: 129
Registered: 13-12-2008
Member Is Offline
Mood: No Mood
|
|
See attached 
Attachment: On Esters of p-toluenesulphonic acid.pdf (502kB)
This file has been downloaded 1578 times
|
|
|
zLo0nGz
Harmless

Posts: 3
Registered: 8-8-2010
Member Is Offline
Mood: No Mood
|
|
Thanks for the artical, Formula409...making isopropyl tosylate might not be an issue but the chemicals used are highly risky, e.g. pyridine and
HCl...we are prohibited from using such chemicals for synthesis purpose as safety is our priority...therefore, simplest way is to react p-toluene
sulfonic acid with IPA, similar to esterification process, distilling off the excess IPA and extracting out isopropyl tosylate...from theory it might
be possible to produce isopropyl tosylate, i need varification...if it is true that by employing this method could produce isopropyl tosylate, is
there any suggestion to purify it? I was thinking of solvent recrystalization method...
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zLo0nGz  | | ...making isopropyl tosylate might not be an issue but the chemicals used are highly risky, e.g. pyridine and HCl...we are prohibited from using such
chemicals for synthesis purpose as safety is our priority... |
I find that an insult to my profession, so you should think twice before posting such nonsense attempts at humiliation of chemists. Working with
pyridine and HCl is bad, but working with isopropyl tosylate is OK? What a bunch of hypocrisy!
Besides, where have you seen that HCl is formed in the synthesis of isopropyl tosylate from isopropanol by using the here described method? The
process Klute describes gives no HCl, just NaCl. Are prohibited to work with table salt as well?
| Quote: | | therefore, simplest way is to react p-toluene sulfonic acid with IPA, similar to esterification process, distilling off the excess IPA and extracting
out isopropyl tosylate... |
And where is a reference claiming such nonsense? How about doing some literature search before spreading misinformation over the internet?
| Quote: | | from theory it might be possible to produce isopropyl tosylate, i need varification...if it is true that by employing this method could produce
isopropyl tosylate, is there any suggestion to purify it? I was thinking of solvent recrystalization method... |
What theory? Have you even bothered checking the theory of esterification before making such a claim? Please explain how does the sulfonic group fit
in that mechanism and how is it supposed to get protonated for the nucleophilic addition of the alcohol to occur. And now you are already asking on
how to purify something that you don't even know what it is. Have you even bothered comparing it on TLC with the starting material?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
| Pages:
1
2
3 |