Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
making ethylene
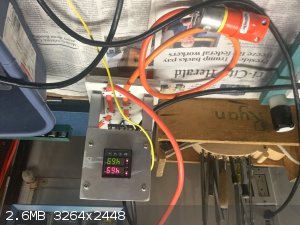
Today I made ethylene using a tube furnace packed with 6" of alumina BBs. The BBs are BASF F200, 1/8" in diameter held in place with 1/8" mesh ss
screens on both ends. The ethanol feed is 95% ethanol (Everclear). The system was first purged with argon. The ethylene was caputured underwater
using the 2 gas bottles shown. When lit the gas burned like a candle with a somewhat sooty flame. I intended to do unsaturation tests with Br2 water
and KMnO4 water but did not have these reagents prepared. The furnace temperature was held at 469°C using a PID controller. The ethanol was kept at
a low boil.
See next post for 2 additional pictures.
[Edited on 1-12-2018 by Magpie]
[Edited on 1-12-2018 by Magpie]
[Edited on 1-12-2018 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Photos are downside up
Great experiment ethylene from everclear 
So if you mixed in silver with the catalysis it seems strait forward to produce ethylene oxide right?
Excerpt
Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst.
[Edited on 1-12-2018 by symboom]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Toggle the pictures. They will invert.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Nice
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This would be a very useful reagent. But how would you integrate the silver with alumina BBs?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
I would probably soak them in AgNO3 solution, bake dry, and heat strongly to decomposition to produce metallic silver and/or Ag2O.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Good suggestion - thanks.
The BBs get heated strongly during furnace heat-up to 469°C.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Nice setup Magpie! Do you plan to use the ethylene in another reaction?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, I hope to make diethyl sulfate using con sulfuric acid.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Nice setup Magpie. It's nice to see some high temperature tube furnace chemistry. This setup is much cleaner than heating ethanol with sulfuric
acid, with lower materials cost and fewer waste products for sure.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks to both of you. Yes, this synthesis seems to be very clean. There was a few ml of liquid in the knock-out pot below the condenser. It did
not smell of unconverted ethanol. Also, I smelled no ether. The run I made was very short.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's the results of the Baeyer test (aqueous KMnO4) and the alcoholic I2 test for unsaturation:

The Baeyer test worked well as the intensely purple solution immediately turned brown. For some reason the I2 test did not work as there was no color
change.
[Edited on 5-12-2018 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
I don't think Iodine is typically able to add across double bonds. In fact, you can dehalogenate a vicinal dibromide with KI in a polar solvent. After
a substitution, I2 or possibly IBr is ejected. Bromine is the best option for an unsaturation test.
|
|
|
jerryhall
Harmless

Posts: 1
Registered: 6-12-2018
Member Is Offline
|
|
Nice setup
|
|
|
wakatutu
Harmless

Posts: 31
Registered: 23-2-2017
Member Is Offline
Mood: Mostly Harmless
|
|
@UC235 - regarding the dehalogenation of vicinal dihalides using NaI in a polar solvent - do you think this is possible to perform on DCM using NaBr
in DCM, leading to the formation of the methylene radical?
|
|
|