| Pages:
1
..
90
91
92
93
94
..
104 |
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Just a WAG (as opposed to a SWAG): could this be some kind of device to deliver 10 mL of a reagent from some kind of reservoir?
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Some time ago I purchased a cheap condenser and it came with an addition funnel. I used the addition funnel for the first time today and it was almost
impossible to get a constant drip rate. Can I replace the tap to get something usable, any tips for getting crappy taps to perform or do I just forget
it?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
For the reaction this is true, but in order to achieve the best phase separation at workup you want the solution to be just barely above freezing IIRC
|
|
|
paulll
Hazard to Others
  
Posts: 112
Registered: 1-5-2018
Member Is Offline
Mood: It's fine. Really.
|
|
Has anybody ever heard of such a thing as a spring-loaded flask clamp? With how handy it would be for receiving flasks it seems to be a thing that
should exist and yet I don't see any anywhere.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by paulll  | | Has anybody ever heard of such a thing as a spring-loaded flask clamp? With how handy it would be for receiving flasks it seems to be a thing that
should exist and yet I don't see any anywhere. |
Could you draw one, just a bad sketch to get the idea?
I'm not sure how that should look like.
I hope not like that: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/produ...
Because that would mean they stole and produced your idea, and that in merely hours!
Those capitalist pigs, no respect on your intellectual property! 
But seriously, like that?
Because initially I imagined a clamp to hold the receiving flask connected with the vacuum adapter(or cow) or such.
Just a plain flask clamp.
Only that I can't picture how such a thing would look like spring-loaded, which is why I was asking for a sketch of it.
And promised, not to steal the idea and sell it to sigma or whoever bids most, you have my hobby chemist word of honor on that! 
|
|
|
paulll
Hazard to Others
  
Posts: 112
Registered: 1-5-2018
Member Is Offline
Mood: It's fine. Really.
|
|
Notwithstanding the fact that I can't draw with or without a computer... something like this. I'm thinking, e.g., for the receiving flask in a
distillation rig - or any other flask that you'd want to change out mid-experiment.
So instead of tightening a thumbscrew to apply pressure to the piece, you'd be unscrewing it to release spring tension onto it, to hold it in place.
The flask could then be removed and replaced by squeezing the other end of the clamp scissors-style. If that makes sense lol
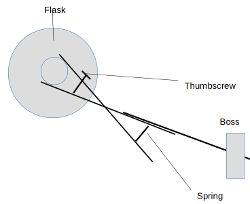
[Edited on 14-9-2020 by paulll]
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Does anyone know a company that makes these cylindrical volumetric flasks?
https://i.ebayimg.com/images/g/QqkAAOSwg35atCrz/s-l1600.jpg
https://i.ebayimg.com/images/g/GLsAAOSwuHZatCr0/s-l1600.jpg
|
|
|
Antigua
Hazard to Others
  
Posts: 155
Registered: 27-9-2020
Member Is Offline
|
|
https://www.wilmad-labglass.com/
Here - I found them through a simple search, but they do look very expensive.
[Edited on 29-9-2020 by Antigua]
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I see some cylindrical volumetric flasks there but not the identical shape. There are also square volumetric flasks I found that are close to it in
proportion such as this one.
https://www.gogenlab.com/lab-supplies/flasks/volumetric-flas...
Just now found it.
https://kinesis.co.uk/dissolution-900ml-volumetric-flask-wit...
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I noticed this 2 liter size is listed for $210.89 and probably shipping costs. I decided to buy that shape having found 4 of them (same Kimble 28040
flask) for sale for $34.25 each or best offer on eBay, new old stock so I bought one along with free shipping. The square flask is 5 X 5 X 18 inches.
https://www.gogenlab.com/lab-supplies/flasks/volumetric-flas...
[Edited on 2-10-2020 by Morgan]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Anyone able to give any enlightenment as to what this reaction is?
https://www.youtube.com/watch?v=cVxySCgUy8A
I thought it was a stunning demo and one I would love to add to my repertoire.
CoCl2 I have. But what is the reaction mixture being used? And what kind of complexing causes the green?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Might be basic peroxide and carbonate? Or basic peroxide and oxalate?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
https://uwaterloo.ca/chem13-news-magazine/march-2014/activities/sharing-chemistry-community-colorful-catalyst
I think this is what you are after.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Awesome. that is just great!
|
|
|
SHADYCHASE54
Hazard to Others
  
Posts: 150
Registered: 16-12-2010
Location: CaNaDay!
Member Is Offline
Mood: No Mood
|
|
Hello I have a quick question regarding how to convert aniline back to actilanilide? I assum it's simple base hydrolysis? Does anyone know of a
decent procedure? I have used TFSE and am coming up with nada. I would rather start from a known procedure then go the route of trail and error as I
only have a small amount of the previously stated starting material; as such I would like to optimize the yield.
|
|
|
Antigua
Hazard to Others
  
Posts: 155
Registered: 27-9-2020
Member Is Offline
|
|
Quote: Originally posted by SHADYCHASE54  | | Hello I have a quick question regarding how to convert aniline back to actilanilide? I assum it's simple base hydrolysis? Does anyone know of a
decent procedure? I have used TFSE and am coming up with nada. I would rather start from a known procedure then go the route of trail and error as I
only have a small amount of the previously stated starting material; as such I would like to optimize the yield. |
Why would it be a base hydrolysis? You have to acetylate the amine, for example using glacial acetic acid and zinc dust.
|
|
|
SHADYCHASE54
Hazard to Others
  
Posts: 150
Registered: 16-12-2010
Location: CaNaDay!
Member Is Offline
Mood: No Mood
|
|
I want to convert the acetanilide back to the amine. My desired product is aniline made by reacting acetanilide, I think with a base, to convert it
into aniline. Can anyone help me with this?
Thank you in advance for any help you may provide.
|
|
|
Antigua
Hazard to Others
  
Posts: 155
Registered: 27-9-2020
Member Is Offline
|
|
Quote: Originally posted by SHADYCHASE54  | I want to convert the acetanilide back to the amine. My desired product is aniline made by reacting acetanilide, I think with a base, to convert it
into aniline. Can anyone help me with this?
Thank you in advance for any help you may provide. |
Now it makes more sense! In your original question you mentioned the other reaction, making acetanilide out of aniline.
Anyways, here are some sources you might find useful:
1. Hydrolysis of acetanilide, attached
2. Reactivity of Acetanilides in the Alkaline Hydrolysis Reaction: Theory vs. Experiment, attached
Attachment: article.pdf (522kB)
This file has been downloaded 895 times
Attachment: Reactivity_of_Acetanilides_in_the_Alkaline_Hydroly.pdf (533kB)
This file has been downloaded 347 times
|
|
|
SHADYCHASE54
Hazard to Others
  
Posts: 150
Registered: 16-12-2010
Location: CaNaDay!
Member Is Offline
Mood: No Mood
|
|
Thanks Antigua, sorry if you found my first post unclear, regardless thanks for the reaction information you rock.
Be well.
|
|
|
Johnny Cappone
Hazard to Self
 
Posts: 74
Registered: 10-12-2020
Location: Brazil
Member Is Offline
|
|
Hi, guys!
I have some questions that I don't think are worthy of a topic of their own. These are as follows:
1) Is it possible to concentrate a diluted solution of nitric acid (say, 10%) to its azeotrope (68%) simply by heating it, as is done with sulphuric
acid?
2) When I distill concentrated sulfuric acid (98%) and KNO3, do I get fuming nitric acid that boils at 83-6 degrees or the azeotrope?
3) If, on the other hand, I use diluted sulfuric acid (30%) in the previous operation, will I get 30% HNO3 or the azeotrope?
Thanks!
|
|
|
Antigua
Hazard to Others
  
Posts: 155
Registered: 27-9-2020
Member Is Offline
|
|
1. Yes
2. Fuming
3. You'd get a solution of HNO3 (less than 68%) that can be distilled fractionally or simply to get the azeotrope.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Johnny Cappone  |
1) Is it possible to concentrate a diluted solution of nitric acid (say, 10%) to its azeotrope (68%) simply by heating it, as is done with sulphuric
acid?
2) When I distill concentrated sulfuric acid (98%) and KNO3, do I get fuming nitric acid that boils at 83-6 degrees or the azeotrope?
3) If, on the other hand, I use diluted sulfuric acid (30%) in the previous operation, will I get 30% HNO3 or the azeotrope?
Thanks! |
1) In a perfect world you would boil of the water at 100 degrees, until the nitric acid reaches 68%, which would then come over at 120 degrees. In
reality, depending on the efficiency of your fractionation, you will start with acid coming over a little bit over 100 degrees, slowly rising in
concentration until it reaches almost 120 degrees.
The efficiency of your fractionation depends mostly on the length of your column and the speed at which you distill the acid.
2) The acid you collect from KNO3 and H2SO4 will be nearly anhydrous, but will contain a lot of NO2. I think adding water to the KNO3/H2SO4 to get
azeotropic acid works better in terms of NO2 produced. You can later make anhydrous acid by adding H2SO4 to the 68% HNO3, preferably distilled under
vacuum.
3) The HNO3 collected from 30% H2SO4 will behave like the acid in procedure 1. You will first collect less concentrated HNO3, followed by acid closer
to the azeotrope.
[Edited on 22-12-2020 by Tsjerk]
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Just want to do a quick check if isopropanol salted-out with NaCl from 70% is safe to distill?
|
|
|
Antigua
Hazard to Others
  
Posts: 155
Registered: 27-9-2020
Member Is Offline
|
|
Why not? Alcohols don't form peroxides like ethers.
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Quote: Originally posted by Johnny Cappone  |
1) Is it possible to concentrate a diluted solution of nitric acid (say, 10%) to its azeotrope (68%) simply by heating it, as is done with sulphuric
acid?
|
The problem is very slope concentration/boiling temperature curve, so it is like most of the acid (4/5 when you start with 50%) goes before azeotrope.
The fraction 119-121 degree will contain 50-55% and only when you get the first signs of NO2 inside the condenser you can collect something around
63-68%.
I think addition of sulfuric acid could help, on rough estimation it keeps 20-30% of water by weight of H2SO4 so it will look like you will starting
with more concentrated acid vapours.
Quote: Originally posted by Johnny Cappone  |
2) When I distill concentrated sulfuric acid (98%) and KNO3, do I get fuming nitric acid that boils at 83-6 degrees or the azeotrope?
|
When NOx is present the boiling point goes down to 80-81%. You can get either red fuming or white fuming nitric acid but the latest one I believe you
can get starting only with azeotrope of high purity (without vacuum). There is a lot of difference between 97.5% and 100% HNO3 (in oxidation power)
and if you don't need it as a rocket oxidiser I suggest get it around 98%. This usually will be result when the equipment, the bottle and the air is
not perfectly dry.
I found that the sulphuric acid changes the boiling curve that after azeotrope the distillation returns back to the lower concentration. And in this
case most of concentration will no go higher than ~45%, it only touches the maximum with few mls of the distillate and then returns back. I think the
sulfuric acid try to keep so much water to become 70-75% sulfuric acid, I think you can roughly estimate the amount you need to add based on this.
[Edited on 24-12-2020 by teodor]
[Edited on 24-12-2020 by teodor]
|
|
|
| Pages:
1
..
90
91
92
93
94
..
104 |