| Pages:
1
..
7
8
9
10
11
..
30 |
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Are amides considered proton/hydrogen acceptors, specifically i was surprised that on this page, http://www.drugbank.ca/drugs/DB00711, diethyl carbamazine was listed as having a hydrogen acceptor count of two?
Obviously one is from the tertiary amine however the other one? Perhaps i am misinterpreting something
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Anybody made any of this material?
http://www.csmonitor.com/Business/Latest-News-Wires/2011/031...
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Can Mg ribbon be used for Grignard reagents or are they vastly inferior to turnings?
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Magic Muzzlet
Hazard to Others
  
Posts: 146
Registered: 22-7-2010
Member Is Offline
Mood: No Mood
|
|
Yes it can, if the metal is of adequate purity. All you need to do is chop it up a bit, then wash it with dilute acid etc etc and it will work. I have
done this.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Good! Turnings seem like a pain to get shipped but Mg ribbons are very common
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
I decide to produce mercury naphthalene sulfonate from sodium naphthalene sulfonate
I want to react mercury nitrate with sodium 1-naphthalene sulfonate(this is good way for produce it?)
I need solubility of sodium 1-naphthalene sulfonate and mercury-naphthalene sulfonate in water
Someone can help me?
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Anybody familiar with this boring process?
Interesting Facts About Platinum (Scientific American article from the 1800's)
'How are they bored?' Ah, sir, you must excuse me that I do not tell you that."
http://chestofbooks.com/crafts/scientific-american/sup3/Inte...
[Edited on 18-3-2011 by Morgan]
|
|
|
12332123
Harmless

Posts: 38
Registered: 14-11-2009
Member Is Offline
Mood: No Mood
|
|
Does anyone have an opinion on whether alcoholysis of aluminium sulphide is a viable procedure for the synthesis of mercaptans (thiols)?
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
You'll get aluminum alkoxide and hydrogen sulfide.
I weep at the sight of flaming acetic anhydride.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Not specifically, but it does remind me of techniques used in
horology (watch-making) for making the holes at the center of tiny gears.
|
|
|
redox
Hazard to Others
  
Posts: 268
Registered: 22-2-2011
Location: The Land of Milk and Honey
Member Is Offline
Mood: Chalcogenetic
|
|
Does anybody know of a minimum-boiling azeotrope that boils at STP? (As if, one liquid is poured into another and the mixture starts boiling)
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by watson.fawkes  | | Not specifically, but it does remind me of techniques used in
horology (watch-making) for making the holes at the center of tiny gears. |
I ran across this article, another very old one.
Invisible Platinum Wire
http://books.google.com/books?id=5KTmAAAAMAAJ&pg=PA193&a...
And this ...
The Fabrication of Ultrafine Platinum Wire
http://www.platinummetalsreview.com/pdf/pmr-v30-i1-027-027.p...
[Edited on 20-3-2011 by Morgan]
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
perhaps this thread should be renamed the short question and answer thread, as i feel people are unaware that questions are posted in this thread so
other people can post the answers.
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
As I was unfamiliar with the platinum wire process, I hoped someone might have some first-hand knowledge of how they do it today, the state of the
art. I mentioned a few things I was able to dig up a few days later after posting the question, but it's still lacking. I was hoping to edge the topic
forward, a thousand pardons.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
My response to that is wtf are you doing on that site?????
lol just kidding but christian and science are as oxymoronic as it comes.
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I was interested in knowing if the new plastic was as good at holding pressure as PET. Every March marks another year my two liter bottles have held
pressure. In 1992 I pressurized these bottles and still today they are hard enough to stand on, still quite taunt - 19 years. At the time I was
planning to make a boat.
As for reading the Monitor, maybe they are starting to come around. ha
"Miller may have gotten the primitive atmospheric composition wrong. But he was right about volcanic plumes. Once again, some of his classic
experiments offer reason to believe that lightning flashing through primordial gases may have provided the energy to form chemical precursors of
organic life."
http://www.csmonitor.com/Innovation/Tech/2008/1028/a-second-...

|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Making Sulfuric Acid,Hydrochloric Acid and copper (I) chloride
I think maybe this more belongs here it is kinda short question
http://www.sciencemadness.org/talk/viewthread.php?tid=15942
|
|
|
12332123
Harmless

Posts: 38
Registered: 14-11-2009
Member Is Offline
Mood: No Mood
|
|
Would DMSO be a suitable alternative to THF for reductive amination of amines with STAB and formaldehyde?
|
|
|
kuro96inlaila
Hazard to Self
 
Posts: 96
Registered: 21-6-2010
Location: Malaysia
Member Is Offline
Mood: Quietly thinking
|
|
These make me confuse:
Yesterday,I decided to conduct a couple of experiments with my sperrylite (PtAs2) mineral.
1.I put about half a gram of PtAs2 in nitric acid.Then I heat it.Sperrylite react with warm concentrated nitric acid producing a yellow solution.Then
I add few milimeter of ammonium chloride solution,hoping that ammonium hexachloroplatinate will be precipitated.But nothing happen.
I guess ammonium hexachloroplatinate only will be precipitated if ammonium compounds solution is added to chloroplatinic acid.While I might have
platinum nitrate in the yellow solution.So,I add magnesium ribbon,hoping that platinum metal precipitated out.It first bubbled,then a brownish orange
compound start to precipitated. Here is the picture of it:

I can't figure what compound it is,so I set it aside and conduct other experiment:
2.I react PtAs2 with aqua regia,then I add ammonium chloride.But no ammonium hexachloroplatinate precipitated.Then I add sodium bicarbonate to the
solution.It first bubbled,releasing carbon dioxide.Then a brownish orange compound start to precipitate.The colour is similar to what was precipitated
in the other experiment.Here is the picture of it:
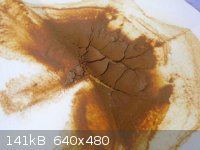
So,what is these thing actually?I guess it would be either platinum dioxide monohydrate or sodium platinum chloride.But I also doubt it is an arsenic
compound.
And,if this is platinum's compound,roasting it should turn it to platinum metal right?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Just so you don't complain that questions don't get answered.
Quote: Originally posted by Panache  | Are amides considered proton/hydrogen acceptors, specifically i was surprised that on this page, http://www.drugbank.ca/drugs/DB00711, diethyl carbamazine was listed as having a hydrogen acceptor count of two?
Obviously one is from the tertiary amine however the other one? Perhaps i am misinterpreting something |
What you are misinterpreting is H-bonds donors/acceptors and confusing them with protonation sites (property of bases). A tertiary amide group, or in
this case a N,N,N',N'-tetralkylurea, is a acceptor of the hydrogen bond. A secondary and primary amide group (or tri-, two- or mono- alkylureas, or
urea itself) are both, donors and acceptors of H-bonds. But unlike amines, they are not bases as far as aq. chemistry is concerned (most amides are
only just about as basic as water, thus protonation in water is nearly negligible).
Similarly, tertiary amines are only H-bond acceptors, but not donors. Ammonium ions, secondary and primary amines, and ammonia, are both, donors and
acceptors (though weaker donors than sec. or prim. amides).
Thus, diethylcarbamazine has two acceptors sites for H-bond formation, but zero donors.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
UKnowNotWatUDo
Hazard to Self
 
Posts: 96
Registered: 30-6-2010
Member Is Offline
Mood: No Mood
|
|
Speaking of amides, maybe you could answer this question also Nicodem (or anyone else I suppose). There is a synthesis I came across that involves
taking a carboxylic acid and a primary or secondary amine and refluxing them in toluene with a catalytic amount of boric acid added (about 0.01mol
eq.). A dehydration reaction follows and the water is collected in a Dean-Stark trap, giving excellent yields of the amide. My question is: in the
case of a low boiling point amine, a gas at STP, how would you modify this reaction to keep the amine in the reaction vessel? Would the carboxylic
acid protonate it to the ammonium salt?Or would you simply be limited by the solubility of your amine in the solvent at reflux temperature? I don't
have any particular examples in mind, this is just generally speaking.
And here is the paper I'm referring to:
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v81...
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I don't think using volatile amines is much of a problem in that type of amidations. Methylamine might be somewhat problematic, but not due to
volatility (you can always put a septum with a baloon on top of the reflux), but because it partitions much better in water compared to toluene, so
lots of it might end up concentrating in the few mL of water collected in the Dean-Stark. This can be surpassed by using some NaOH pellets in the
Dean-Stark to absorb the water as it collects. But then again you don't really need an excess of the amine in that procedure so the partial pressure
of any amine, including the volatile ones, will be very low in the vapour phase (ammonium carboxylates do dissociate easily, but the equilibrium in
most cases does not allow for much free amine).
I used these boric acid and phenylboronic acid catalysed amidations a few times and I must say they work like a charm in most cases. I highly
recommend these methods over any coupling reagent whenever applicable (when using robust substrates). There are plenty of other references, besides
that Org. Synth., by the way.
|
|
|
UKnowNotWatUDo
Hazard to Self
 
Posts: 96
Registered: 30-6-2010
Member Is Offline
Mood: No Mood
|
|
Thanks for the two cents, it's much appreciated.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Will pyrogallol reduce copper?
Will pyrogallol in aqueous solution reduce Cu++, and if so, will it reduce it to Cu+ or all the way to elemental copper? Is it pH dependent?
|
|
|
experimenter
Harmless

Posts: 19
Registered: 7-11-2010
Member Is Offline
Mood: No Mood
|
|
Chlorination with CuCl2
Trying to perform chlorinations using CuCl2 in microwave oven, I stumbled across the following observation:
sodium acetate in water, when reacted with cupric chloride (CuCl2) gave a white precipitate (CuCl I suppose). This means that a reaction happened but
I highly doubt that it chlorinated the acetate (in order to give the sodium monochloroacetate).
The ph of the solution after reaction was around 5.
What do you think, is it possible that CuCl2 can chlorinate the sodium acetate? (supposedly CuCl2 can chlorinate carbonyl compounds)
I know about the usage of LiCl catalyst, but in that case, it wasnt needed, thats why I'm suspicious that chlorination hapenned.
|
|
|
| Pages:
1
..
7
8
9
10
11
..
30 |