| Pages:
1
..
82
83
84
85
86
..
104 |
Volitox Ignis
Hazard to Self
 
Posts: 53
Registered: 28-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  |
Vinyl will probably work. Allyl and aryl ones definitely do. Alkynylmagnesium halides would not be make by the reaction of a 1-haloalkyne with
magnesium, but by the reaction of a 1-alkyne with another Grignard (acid-base metathesis). |
In the case of acetylene, will that produce Ethynyldimagnesium dihalide (C2Mg2X2) or just ethynylmagnesium halide
(C2HMgX), or both?
[Edited on 27-3-2018 by Volitox Ignis]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Depends on whether or not you use an excess of acetylene.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
How to determine sodium percarbonate purity ?
I just received 2 kg Sodium Percarbonate, for laundry and chemistry.
https://www.ebay.co.uk/itm/SODIUM-PERCARBONATE-OXYGEN-BLEACH...
Assuming that it is mostly sodium percarbonate,
possibly with excess sodium carbonate and 'coated' (with what I do not know yet)
I would like to know what is the actual H2O2 content w/w.
I'd like a quick and easy method for now,
and maybe a procedure for precise determination later.
So, what is an easy method of determining the H2O2 content of 2Na2CO3.3H2O2 +
'other' ?
At the moment all I can think of is titration vs. dextrose with methylene blue indicator.
[Edited on 28-3-2018 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
My first approach would be to do an iodometric titration.
I don't know for sure if that will get you the information you need. But knowing how much oxidative power you have per gram is not a bad thing to
know.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I thought that the sodium carbonate would react with iodine to form iodide and/or iodate ?
Would MnO2 decomposition of H2O2 be interfered with by the sodium carbonate ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
And the H2O2 reacts iodide to iodine.
Good point -- there's going to be a couple of equilibria in action here and it might not play nice.
You might try a thermal decomposition and see how much CO2 and H2O2 is given off...
I am just thinking out loud here. There are likely problems with that too and not just the high temperature required.
|
|
|
Volitox Ignis
Hazard to Self
 
Posts: 53
Registered: 28-1-2016
Member Is Offline
Mood: No Mood
|
|
How can I determine whether a sample contains 1,2,3 trichloropropane?
How can I properly dispose of it?
What are the symptoms of exposure to it?
[Edited on 7-4-2018 by Volitox Ignis]
|
|
|
Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Quote: Originally posted by Volitox Ignis  | How can I determine whether a sample contains 1,2,3 trichloropropane?
How can I properly dispose of it?
What are the symptoms of exposure to it?
|
I can only answer the last question by citing wiki:
| Quote: |
Humans can be exposed to TCP by inhaling its fumes or through skin contact and ingestion. TCP is recognized in California as a human carcinogen, and
extensive animal studies have shown that it causes cancer. Short term exposure to TCP can cause throat and eye irritation and can affect muscle
coordination and concentration. Long term exposure can affect body weight and kidney function. |
we apologize for the inconvenience
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Answering my own question :)
I just decomposed sodium percarbonate in solution using MnO2
(cleaned MnO2 with carbon from a lantern battery)
The O2 yield was 106%
As it was a quick small scale test setup with no calibration
I can live with the result and consider it as 100% sodium percarbonate.
Sorry to answer my own question but as a cheap source of H2O2 I thought others may like to know that
at least one sample of eBay sodium percarbonate is 100% (approx.)
----------------------------------------------------------------------------
I ignored the possibility of the carbon being oxidised as the molar volume of CO2 would be the same as any used O2.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Could you please elaborate on what you did to find the concentration ?
Sounds like Chemistry to me !
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
more thought process than the actual trivial chemistry
A little research quickly identified the most likely contaminant/diluent in commercial sodium percarbonate to be sodium carbonate,
so a simple acid:base titration may give confusing results.
As I have a jar of MnO2 (mixed with Carbon powder) from a previous 6v lantern battery dissection,
using that seemed a simple choice.
Decompose the hydrogen peroxide - measure the volume of gas produced.
As above, I decided that if carbon would interfere in some way
then it would probably be by reducing/consuming some of the oxygen produced,
but as each mole of O2 consumed would produce one mole of CO2,
the gas volume produced should be the same - carry on but keep in mind.
I ignored CO2 solubility.
As I only wanted to know the available peroxide content for use in future experiments,
which are not likely to be precisely stoichiometric, I did not intend to try for precision.
The only bit of chemistry is;
2Na2CO3.3H2O2(s) => 2Na2CO3(aq) + 3H2O2(aq)
2H2O2(aq) => 2H2O + O2
So 1 mole of 2Na2CO3.3H2O2 produces 1.5 moles of O2
314g of sodium percarbonate to produce 33.6 litres of gas. (1 atm. 0oC)
For a quick test, I chose bubbling gas into an inverted measuring cylinder to measure gas volume,
my largest is 100ml,
so scaling down gives 0.9345g for 100ml gas at STP.
I decided to ignore STP compensation as my shed is still near STP 
About 0.9g should be OK.
Procedure
Tare a 250ml Erlenmeyer flask with side arm and add approximately 0.9g sodium percarbonate, record actual weight added as M1 (g).
Add c100ml warm water to dissolve the sodium percarbonate.
Fit a rubber tube to the side arm and put the other end of the tube in an inverted 100ml graduated cylinder filled with water, which is clamped above
a 1l beaker with c500ml water.
Add catalyst and quickly stopper the flask.
(Catalyst = cleaned mixture of MnO2 and carbon powder from lantern battery, less is required I guess if pure MnO2 is used)
The equipment setup should look something like this edited plagarised drawing
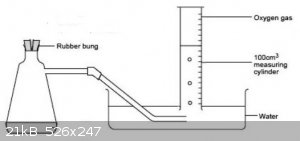
When the bubbling rate subsides, swirl the flask to ensure completion of peroxide decomposition.
When all bubbling has ceased, record the volume of gas collected as V1 (ml)
Yield = (V1/M1) x (314/33,600) x 100 (percent)
In my case, M1 = 0.89, V1 = 101
Actual yield = (101/0.89)x(314/33,600)x100 = 106%
Notes;
. as my lab is at 197m above sea level and was slightly warmer than 0oC, the extra 6% is easily accounted for.
. the scales used were 300g x 0.01g resolution, reliable to +/- 20mg when stable and calibrated, so a source of up to 2% error.
. to fill an inverted cylinder the tube can be inserted up to the top of the inverted cylinder and remove the air by suction.
(I knew that my tubing was clean so I just sucked the air out using my mouth, it would be wiser to use an air pump)
. I recorded 101ml using a 100ml cylinder ... the extra '1' is estimated.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Nice piece of work. This is the kind of practical chemistry I like.
I will test my own sample of sodium percarbonate in this way.
The compound is not a true percarbonate, it actually is a normal carbonate, with hydrogen peroxide in the crystal lattice. We all know water of
crystallization. Here you have a compound with hydrogen peroxide of crystallization instead of water of crystallization.
True percabonates also exist, they contain the CO4(2-) ion, which has a resonance structure of a central C-atom with three oxygens attached, where one
of the three oxygens has another oxygen attached. Two of these O atoms have appr. 1.5 bond with the C atom, the one with the fourth O-atom attached
has a single bond with the C atom. True percarbonates, however, are not easily obtainable.
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Does Nickel (II) Chloride Hexahydrate hydrolyse upon heating? Can i just heat it to get the anhydrous salt?
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
To test my diethyl ether for peroxide I intend making KI/starch papers,
to verify a negative result I need to check the papers with some other oxidizer.
A quick google did not reveal the ionisation potential of diethyl ether peroxide,
so
Someone please suggest an oxidiser to test diy KI/starch papers that is less oxidising than diethyl ether peroxide ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
From wiki:
Heating the hexahydrate in the range 66-133 °C gives the yellowish dihydrate, NiCl2 · 2 H2O.
The hydrates convert to the anhydrous form upon heating in thionyl chloride or by heating under a stream of HCl gas.
Simply heating the hydrates does not afford the anhydrous dichloride.
NiCl2 · 6 H2O + 6 SOCl2 -> NiCl2+ 6 SO2+ 12 HCl
The dehydration is accompanied by a color change from green to yellow.
Quote: Originally posted by Sulaiman  | To test my diethyl ether for peroxide I intend making KI/starch papers,
to verify a negative result I need to check the papers with some other oxidizer.
A quick google did not reveal the ionisation potential of diethyl ether peroxide,
so
Someone please suggest an oxidiser to test diy KI/starch papers that is less oxidising than diethyl ether peroxide ? |
I think it would be easier to just add some reducer to your diethyl ether, and keep it there, so no peroxides could form. Also - hide it from light.
If you need the oxidizer - it is a longshot. What do you have? 
[Edited on 17-4-2018 by TheMrbunGee]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by TheMrbunGee  |
Heating the hexahydrate in the range 66-133 °C gives the yellowish dihydrate, NiCl2 · 2 H2O.
The hydrates convert to the anhydrous form upon heating in thionyl chloride or by heating under a stream of HCl gas.
Simply heating the hydrates does not afford the anhydrous dichloride.
|
Weird. I thought nickel chloride is one of the few transition metal salts that does dehydrate fairly well with just heating.
It can also be dehydrated in ethanolic suspension by heating with triethyl orthoformate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Sulaiman  | To test my diethyl ether for peroxide I intend making KI/starch papers,
to verify a negative result I need to check the papers with some other oxidizer.
A quick google did not reveal the ionisation potential of diethyl ether peroxide,
so
Someone please suggest an oxidiser to test diy KI/starch papers that is less oxidising than diethyl ether peroxide ? |
I don't know about less oxidizing, but you can test them with bleach.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I have many oxidisers that should give a positive result with KI/starch paper,
my concern is that my diy test papers react to say H2O2 but not any ether peroxide actually present,
because I have no idea what the redox potential of diethyl ether peroxide is.
I do not expect problems as my diethyl ether has been stored in shade in a brown bottle with little headspace, it may even contain an inhibitor.
I want to try the test more from curiosity than fear,
but I am motivated partially by safety concerns due to my inexperience.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by Sulaiman  | I have many oxidisers that should give a positive result with KI/starch paper,
my concern is that my diy test papers react to say H2O2 but not any ether peroxide actually present,
because I have no idea what the redox potential of diethyl ether peroxide is.
I do not expect problems as my diethyl ether has been stored in shade in a brown bottle with little headspace, it may even contain an inhibitor.
I want to try the test more from curiosity than fear,
but I am motivated partially by safety concerns due to my inexperience. |
Maybe try some nitrates? Not really sure, just try them..
|
|
|
RogueRose
International Hazard
    
Posts: 1592
Registered: 16-6-2014
Member Is Offline
|
|
"Sugar mix" for protien shakes - milk replacement - need to figure out ingredients
I've been trying to figure out the proprietary composition of a sugar sweetener used in a protein shake I used to make at my job. It was a mix of 2
white powdered sugars that I'm 99% sure were not standard table sugars or other easily available sugars at the grocery store. We were supposed to mix
the powder with water, then blend to mix. then allow to sit in fridge until it was needed to make the shake.
Making the shake was adding the protein flavoured stuff and then filling with the sugar liquid that looked like milk. These were the best tasting
shakes I've ever had and all the customers agreed and it was some relatively unknown company (apex) that only supplied some gyms/fitness centers - not
available at retail stores but could buy in gym and it was expensive (and they didnt' sell the sugar mix).
The odd thing is that if you made the sugar mix, allowed to sit in fridge for 7-10 days, it smelled SOOOO bad it would make you wretch. This wasn't
b/c the containers were dirty or the blender, I cleaned them well, soaked in bleach (diluted w/ water) for 2 hours, then scrubbed with a scotch brite
scrubby the entire thing, so it had to be almost completely clean. But I'll never forget that smell.
I think I remember the names like Maltose, I know there was a "dextrine" maybe a maltodextrine and dextrose are the two I think I remember from the
packaging. I'm pretty sure it is the last two of these. I do remember when I looked up prices for these back in 2000-2004 they were VERY high
compared to sucrose, like 10-20x higher. We also were chastised for using too much as we were told it was expensive and to "cut it with water"... but
we didn't use to much , it just spoiled but no manager wanted to hear that or ever smell it to confirm. I hate the man sometime!!.
So even now when I research dextrose I get a link to Glucose in Wiki. I know there was something "maltoXXXX" and pretty sure it was maltodextrine.
we were told we were making basically a "sweet milk" that gave instant energy. Are there any other "MaltoXX" based sugars that I could be mistaken
for this one?
[Edited on 4-29-2018 by RogueRose]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Dextrose and glucose are the same thing. I know maltose is a separate sugar, but that's about the extent of my carbohydrate knowledge.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
I've been searching to find if the Dakin West reaction will produce a ketone from PABA...but this far i haven't found anything in the literature,
perhaps i haven't posed the question correctly....any suggestions,... solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
User13579
Harmless

Posts: 14
Registered: 27-2-2018
Member Is Offline
Mood: No Mood
|
|
From looking at the reaction mechanism I don't think it'll work with a benzoic acid. You need a C-H adjacent to the carboxylic acid, which benzoic
acids don't have. There are probably other methods, but I can't think of one using simple chemicals right now. Perhaps you can react aniline with 2
equivalents of acetyl chloride or acetic anhydride with a Lewis acid catalyst? Although aniline sometimes forms a dimer under similar conditions so
I'm not sure that it would give good results.
[Edited on 9-5-2018 by User13579]
[Edited on 9-5-2018 by User13579]
[Edited on 9-5-2018 by User13579]
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
When determining R, S chirality centers, is it required to have the hydrogen pointing at you and go from largest substituent to smallest or can I
point the hydrogen away and go from smallest to largest?
I haven't encountered a molecule where the first nethod is more beneficial than the second.
The philosophy of one century is the common sense of the next.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by ninhydric1  | When determining R, S chirality centers, is it required to have the hydrogen pointing at you and go from largest substituent to smallest or can I
point the hydrogen away and go from smallest to largest?
I haven't encountered a molecule where the first nethod is more beneficial than the second. |
Always point the hydrogen (or lowest priority group) to the back, and go 1, 2, 3. it's not "largest", but according to atomic number, by the first
point of difference. So -F would outrank -CH2CBr3
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
..
82
83
84
85
86
..
104 |