| Pages:
1
..
78
79
80
81
82
..
104 |
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
@RogueRose I'm convinced that you live in a junkyard. IIRC, lead-acid battery chargers are often able to output at both 6V and 12V, and are probably
the best power source for this type of thing. Every one that I've seen has an ammeter on it too. If you know any diesel mechanics, they'd be
guaranteed to own a decent one, and would probably let you borrow it if you did them a favor. Same goes for anyone that owns a bulldozer or front-end
loader or a lot of that type of heavy machinery. Your stainless steel cathode is probably not a good idea. Stainless steel is protected with a
chromium oxide layer, and the cathode reduces things. All that in a chloride-rich bath just seems like a bad idea. They don't make "cathodized
aluminum" for a reason, after all. Try copper or graphite, maybe? That way you could tell by the change in color if any lead was dissolving and
plating.
@ninhydrin1 Yes. You could even use Vasaline if temperatures didn't get too high. Silicone is used mainly because its viscosity doesn't change much
with temperature.
[Edited on 11/9/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Negative X
I watched a King of random offering recently https://www.youtube.com/watch?v=I7vXvLvowgI. (It appears that Grant Thompson now has a side-kick.)
This video has a nice thermal mix of zinc metal, ammonium nitrate and ammonium chloride that is quite unstable. And of course it has a suitably
hyperbolic but nondescript name. The guy appears to understand little chemistry but he makes a reasonable stab at presenting some chemical equations
so that is at least something. (I haven't bothered working out the stoichiometry of his mix.)
The question I have, is why the green? The flame colour seen is not what I would normally associate with zinc. Anyone have any insight?
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
According to Thompson's Illustrated Guide, the flame test for ammonium ions is a faint green but is often masked by other species. That might explain
it. The ammonium nitrate decomposes while the ammonium chloride present burns in the flames produced to form a green colored flame.
The philosophy of one century is the common sense of the next.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Chlorine can make a flame green. And nitrate could oxidize chloride to chlorine.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
I can personally report that one of the few pyrotechnic star compositions using Zn as a fuel (Weingart's "granite stars", AKA Zinc spreader stars)
burns with a green flame.
The mixture is Zn fueled and KNO3 oxidized, so I think we can leave ammonium spectra out of the consideration?
(Edit)
Of course, S will react with Zn as well... Anyone recall the good old Zn/S rocket fuel flame color? Have not seen that burn since the 1970s.
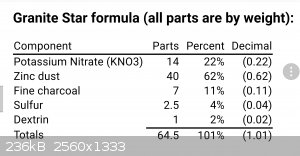
[Edited on 11-11-2017 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
That's cool. I have only associated blue/white with zinc flames. Which is to say therd is a whole lot more going on in the spectra than the elementary
textbooks suggest.
|
|
|
RogueRose
International Hazard
    
Posts: 1592
Registered: 16-6-2014
Member Is Offline
|
|
Diatomaceous Earth washed with HCl - Using the liquid for FeCl2?
I used some HCl (hardware store Muriatic - which is very clear and clean it seems) to clean some Diatomaceous Earth and the result was very yellow
liquid and I think it still has a good bit of HCl and is not completely FeCl2. So I was wondering if this can be used to make some FeCl2 - or is
there a good chance that there is other metals dissolved in the liquid. The DE was a high quality garden variety that is much whiter than many DE's
I've seen so I think it was fresh water DE as opposed to salt water DE which tends to be darker and have more metal contaminates.
I'm not sure what else to do with the liquid and if there is something else that it could be useful for, I'd appreciate any input on that. Thanks!
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
Hello everyone. I am considering purchasing a thermocouple. I found some relatively affordable ones on eBay. I found the thermocouple reader w/ 2
extra probes, an additional probe that claims to have a temperature range of -100 to 700 degrees Celsius, and a thermometer well for just over 25
bucks. What I am asking is if it is worth it. I am a broke teenager so I don't want to spend anymore money than I have to  . For those of you that use these, do you prefer a standard glass thermometer or do
you like your thermocouple better? . For those of you that use these, do you prefer a standard glass thermometer or do
you like your thermocouple better?
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Thermocouples with digital readout are easier to read than conventional thermometers but not as accurate unless calibrated
Can anyone tell me,
What is a quick reliable easy method to differentiate between hdpe and PTFE ?
(I have no fluorine compounds, but many common solvents)
[Edited on 16-11-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
OldNubbins
Hazard to Others
  
Posts: 136
Registered: 2-2-2017
Location: CA
Member Is Offline
Mood: Comfortably Numb
|
|
Based on their densities, I imagine PTFE would sink in water while HDPE floats.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Shave a small sliver of the plastic off, hold it in a lighter flame. HDPE will easily melt and then burns like parafin with a smell like a wax candle.
PTFE does not burn in air, it will just degrade and smells quite different. Don't sniff too deeply or often of overheated Teflon.

Attachment: Plastics identification.pdf (1.4MB)
This file has been downloaded 946 times
[Edited on 16-11-2017 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Sounds like UHMW plastic sheet, lots of uses. Essentially solvent proof, non stick- almost no adhesive will bond, but can be thermally welded. It's
self lubricating, tough, can usually be tapped for threaded holes. Makes a good sliding base for saws or routers to be run on surfaces that metal
could mar, my usual use.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Thanks for the quick responses,
Today a workmate gave me a 1" 24" x24" slab of white plastic that he thought may be ptfe,
I just tested it and unfortunately it burns nicely with a blue flame, dripping screaming blobs of molten plastic 
Almost certainly hdpe/uhmwpe, which is still useful.
EDIT: Sorry Bert, this post was before yours, but I tried to edit it and messed up so posted it again, by which time you posted the above ... .
I use hdpe quite a lot for stupid-high-voltage stuff, almost perfect - until it melts.
[Edited on 16-11-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by Sulaiman  | Thanks for the quick responses,
Today a workmate gave me a 1" 24" x24" slab of white plastic that he thought may be ptfe,
I just tested it and unfortunately it burns nicely with a blue flame, dripping screaming blobs of molten plastic 
Almost certainly hdpe/uhmwpe, which is still useful.
EDIT: Sorry Bert, this post was before yours, but I tried to edit it and messed up so posted it again, by which time you posted the above ... .
I use hdpe quite a lot for stupid-high-voltage stuff, almost perfect - until it melts.
[Edited on 16-11-2017 by Sulaiman] |
You do realize 1" of UHMWPE will stop most conventional firearms, right? That's an armor plate you've got right there, and an expensive one at that -
I'd give an arm and a leg to have that much UHMWPE at one time.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
Could benzyl nitrate be prepared via nitration of benzyl alcohol,
or is the aromatic ring likely to be nitrated to, forming a mixture of nitrobenzyl nitrates?
Benzyl nitrate doesn't appear to be very well documented, but from what I can find it seems to be a liquid that's fairly stable at room temperature.
reality is an illusion 
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
Would a paint can hold up against plante1999's synthesis of TiCl4, as quoted below (assuming it actually works)? If not, what is a suitable container
for this reaction (which will take place at around 400 degrees Celsius)? I will be using a mini butane burner I happen to have.
Quote: Originally posted by plante1999  | Simply melt pyrosulphate with the ore in presence of NaCl, it will make HCl + TiCl4
Sources: my own work
In fact I already made most inorganic titanium compounds and many organic ones, I think that I have some(a lot) knowledge in this field.
[Edited on 17-5-2012 by plante1999]
[Edited on 17-5-2012 by plante1999] |
EDIT: Added clarifications.
[Edited on 11-22-2017 by ninhydric1]
The philosophy of one century is the common sense of the next.
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I was needing a rubber stopper so I went to my box of neoprene rubber stoppers with each size grouped in a Ziplock bag. But they have become oily and
was wondering what's going on with that? What might the oily substance be?
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
What is the name of the theoretical sieve that reverses entropy by allowing only high-energy molecules to penetrate while elastically bouncing off
low-energy molecules?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Maxwell's demon?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I think that's it. I remember hearing it described as something like a bucket that leaks boiling water as the water inside freezes.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Larry Niven's "Unfinished Story #1" (or #2) involved the idea.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Can sodium pyrosulfate be used as a dehydrating agent similar to, say, anhydrous copper sulfate?
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
Does anyone know of a good way to distinguish rhenium and tungsten? I bought some cycloid tube filaments on ebay advertised as rhenium, but I've been
having doubts on their authenticity.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Well, tungsten will react with H2O2 to form tungstic acid which is a pretty yellow-coloured solid. Tungsten is also quite dense with a specific
gravity of 19.3. I don't know if either of these properties is sufficient to ditingush it from rhenium but that is where I would start.
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
I've gotten that far: rhenium also reacts with hydrogen peroxide to form perrhenic acid, while tungsten forms a soluble complex in excess peroxide.
Rhenium is also denser than tungsten, so that rules that one out.
I do notice that some of the strips have an iridescent tarnish on them. Is this a bad sign?
|
|
|
| Pages:
1
..
78
79
80
81
82
..
104 |