| Pages:
1
..
6
7
8
9
10
..
48 |
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Ouch, I had a bad feeling they'd be extraordinarily expensive. That's more than twice the cost of the most exotic process electrode I've seen so far.
I wrote him as well. He's probably wondering why all these people are asking him about the chlorate process. The next thing you know, he's googling
"Reflex Electrode" and finding our secret lab here.
Glad you finally got the anodes.
As for pH, I think a calculated dose combined with an occasional measurement will suffice. Unless I am totally wrong about the poisoning, and a
modest double-junction electrode costing $70 will last a year or two... that'd be worth it.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Update: I promised in my blog that my "T-Cell" would be running... yesterday. It's still not running, for one primary reason. I had ordered a
dosing pump off eBay, but I was not expecting it for several days yet. it arrived early, and now I am cramming, trying to integrate it. I don't
think it's going to happen.
The pump: A Hanna BL5. http://www.hannainst.com/usa/prods2.cfm?id=018001
I made a big mistake. I placed the order before I really thought about the volume of acid to be delivered (low) and the fact that I was planning on
automatic pH control, rather than semi-manual. This pump delivers FAR too much with each stroke.
Benefits: The BL pumps can be bought bew off eBay for a hundred bucks. Good dosing pumps are normally $300-$500. Everything wetted is either kynar,
PTFE, or glass, good for darned near anything, chemical wise. And the interesting thing, with each stroke of the pump, exactly 1 ml is delivered. As
far as I can tell, it is quite precise.
This means for 50 amps, I'll need about 4 strokes per hour. When the pump is set on it's slowest speed, it executes one stroke every 5 seconds. This
means that each hour, unless I use a controller, the pump would need to come on for 20 seconds, then shut down. I don't have a timer that will do
that. The average consumer timer would come on for at least one minute.
I could dilute the acid by three, OR, I could have it come on for a minute, overdose slightly, then shut down for three hours.
Anyway, I need to figure out how to do the acid additions. Maybe I should set up a drip while I figure out how to integrate the pump.
Lesson learned... if you shop for a dosing or chemical pump for a home chlorate system, get one that delivers very small dosages; or, one that can be
minutely controlled.
I hope to get the system running tonight or tomorrow morning. I'll need at least 24 to 48 hours of nearly cotinuous monitoring and tweaking to ensure
there are no leaks, the temps are good, and the system is behaving as it should.
It has really gotten more complicated than I had anticipated. Oh well!  It's
also been a lot of fun! I just hope all the work and materials are not going to go to waste. It's
also been a lot of fun! I just hope all the work and materials are not going to go to waste.
My old system with no pH control was 60% efficient. I think I can get this to 80% or better, meaning at 80 amps (if it'll handle that without
overheating, the projected yield would be 1.46 kilos per 24 hour period at 100%, and 1.10 kilos at 75%, with an estimated capacity of 25 liters.
My old system of 6 liters was doing 1 kg per batch from 14% down to 7% chloride, so this rig should do at least 4 kilos per run. If I can take it to
3% chloride, it should do 6.8 kilos. Since I am hopelessly optomistic with estimates, a more realistic value is in fact 3 to 4 kilos max. Can't wait
to fire it up!
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Sorry for the string of posts... it's running, and everything so far is perfect. No problems at all. 50 amps created a delta T (temperature
differential) of 20 degrees within 15 minutes. The pump is barely ticking over right now. If the DT gets too large, I'll crank the pump up a bit.
pH within minutes was at 8.4. Until I can rig up a drip (probably tomorrow) I'm simply ging to inject some HCl using a syringe.
Electrode spacing and area looks good... 5V on the supply created a current of 55 amps. If everything goes well, and the system doesn't overheat, I
may try 70 or 80 amps.
I'll get some pics up later. 
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Nice news! Why not setup the dosing pump with a tee and a couple of kinks/knots to divide the flow, and divert most of the flow back to the HCl
container? The pump will deliver sufficient pressure to keep the flow rate reliable (you certainly aren't going to get crap growing in the solution).
Pretty f'in sweet that your 5v delivers just the right level of current.
[Edited on 4-11-2008 by tentacles]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Good to see machine up and cranking.
One small note. If you happen to pump far too much acid into the electrolyte and put the pH way down, the cell will start to produce Cl<small>2
</small>gas. This may be a serious problem if you have it in a place where the gas cannot vent off safely, so be careful with the acid.
I presume you are using KCl?
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Tentacles - great idea! I didn't even think of diverting the flow. I'll try to set something up tomorrow.
Dann2, the system pipes to the outside, no fears about gas. Everything looks tight and dry. Just a little bit of salt creep on a couple of threaded
joints, otherwise, all the seals are doing well. Yes, I'm using KCl, plus some used electrolyte from previous runs, recharged with chloride.
The pump and temperatures are behaving exactly as I wanted them to. The actual flow through the EC is probably no more than 1 liter/hour or less,
it's just a thin stream. As the pump rate goes up, the DT goes down, and of course if the pump is backed off, within a few minutes the DT rises. The
CPVC electrode cell is at 60 degrees, and the big chamber right now is at 35.
The cathode pair is barely warm. The anode, a bit hotter. I'll probably take a few minutes tomorrow and bolt a heat sink onto it. Nothing extreme,
it's probably 80 degrees C or so.
The cheap pH electrode is working well so far. I'm doing manual checks, drawing the liquor hot from the EC, and it's tending to hang around 8.0. I'm
limiting contact of the pH probe to the nasty electrolyte, and washing it clean immediately. I've been light with the acid so far; I'll add a bit
more.
[Edited on 4-11-2008 by Swede]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Swede, is that the same size shank as you installed on mine (it looks like it)?
Did you get just one cheap probe? I'm really curious to see how long they'll hold up in continuous use. At $15 a pop, even if they only last a month
or two of use, that's really not too bad at 2lbs a day. Hopefully we'll be surprised by their longevitiy - I know I've already been surprised at low
long DSA last in commercial use (more than 3 years).
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Tentacles - The shank is identical, 0.041" thick by maybe 0.950" wide. It's doing 50 amps with ease. The aluminum block connector helps a bit. I
may clamp a heat sink to the block, and later attach it permanently, but it's really not needed.
pH - when I bought the dosing pump from an eBay outfit called eSeasonGear
http://stores.ebay.com/eSeasonGear
I picked up the electrode as well. I'd say it is not a super-cheapie, but it is definitely low on the totem pole. $37. It is a double-junction
electrode from Milwaukee instruments...
http://cgi.ebay.com/ws/eBayISAPI.dll?ViewItem&item=28017...
It is the yellow one in the picture. The first thing I did was prepare a liter of electrode storage solution using KCl, and I soaked it overnight.
The next day, I calibrated the Extech controller using pH 7.00 and 10.00 buffers. I was really happy to see 7.01 and 10.01 on the meter without
having to touch the set screw.
The way I am measuring pH right now is simple. I draw off a few ml of liquor from the ball valve on the exit port of the EC... comes out quite hot.

The electrode is rinsed, goes into the liquor for maybe 2 seconds, I note the reading, and out it comes immediately into distilled water for a rinse.
Then, right back into storage solution. With such limited contact to the liquor, I am betting it will last a long time.
One thing I've noticed in the 16 hours it's been running... the pH is not responding as well as I'd like to additions of HCl. I started by dosing 4ml
conc. HCl per hour. The pH would go from 8.2 to 7.9, but fairly quickly go back up to 8.2. It seems to want to hang there at 8.2, as if it is
buffered there.
Today I am going to force the issue with much larger HCl additions. If I can get it below 7.0, I am going to see how long it will stay there. If it
climbs back quickly to 8.2, then I think I can conclude that manual, periodic acid additions, while helpful, probably won't be able to keep the pH at
6.6 or 6.8. That would require true control; or, a continuous drip or dosing setup.
This begs the question again of buffering with chromates, or the phosphate ions. I'd really like to give the latter a try. I will conclude this run
with no buffers, but for the next one, I'd definitely like to find some sodium phosphate salts and try the buffering as described in the patent.
[Edited on 5-11-2008 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Just a though. Would the sample you are taking for pH measurement be coming from close to the Cathode. It may show too high a pH if so.
You should be taking a sample from the solution bulk. The bulk pH should be at 6.6 to 6.8 for the 'Chemical Chlorate formation' reactions(s) to happen
in the bulk area.
Perhaps you could measure the pH of the crystallization chamber to see what that is. It may be a better indication of the cell bulk pH.
Keep checking calibration of probe.
Make sure the sample has cooled before you measure (or is probe ATC?).
Remember that ALL stuff that you have read about pH control is in relation to Sodium Chlorate cells (not K). Perhaps pH control of K cell is not so
simple or perhaps it is inclined to run at a higher pH. I would be inclined to think that a K cell would be similar to a Na cell but it's possible
that they are different.
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Good news, with some moderate HCl doing it's down to a more realistic 7.5. And good comments too re: bulk cell pH. I'll pull some samples from there
and check them. I was not aware that the bulk of the reactions take place away from the electrodes.
I recieved an overnight order from MSCdirect, enough plumbing stuff to hook up the dosing pump. I have a hardware store timer, and with no bypass,
it'll administer 12ml in one minute. From what I've seen so far, that is a reasonable dose every three hours. I'm close to having a hands-off setup!
The initial chloride content BTW was 145 g/l. Unless something odd happens, I'm going to take it to 50 g/l this run, perhaps lower in future runs.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Swede
Good news, with some moderate HCl doing it's down to a more realistic 7.5. And good comments too re: bulk cell pH. I'll pull some samples from there
and check them. I was not aware that the bulk of the reactions take place away from the electrodes.
The initial chloride content BTW was 145 g/l. Unless something odd happens, I'm going to take it to 50 g/l this run, perhaps lower in future runs.
|
Hello,
It's not really 'the bulk of the reactions' but rather 'the bulk' reactions. I know it sounds ridiculous to bring it up but there is a difference.
This is why, when we are controllig pH, it is good to have a seperate chamber where the 'chemical Chlorate formation reactions' (also called the 'bulk
reactions') can take place, away from the electrodes. (Or you can have a single, large, chamber which contains the electrodes + this chamber will have
volume away from the electrodes, known as the 'bulk').
When controlling pH is not great to have a small 'bulk' area in the electrodes chamber. Large chamber in relation to electrode size is best (or a
seperate, very warm chamber).
When we are not controlling pH, it does not matter a toss about the size of electrode chamber in relation to electrode size (within reason) as there
is NO 'Chemical Chlorate formation reactions' taking place. Chlorate is make by a reaction called 'Anodic Chlorate formation'. The vast majority of
garage Chlorate cells (no pH control) make there Chlorate by 'Anodic Chlorate formation'.
'Anodic Chlorate formation' is avoided like the plague in industry (pH controlled used!) as it is considered an electricity robbing reaction.
Since you are doing pH control you should perhaps aim for this lofty ideal! (seperate 'chemical Chlorate formation' chamber)
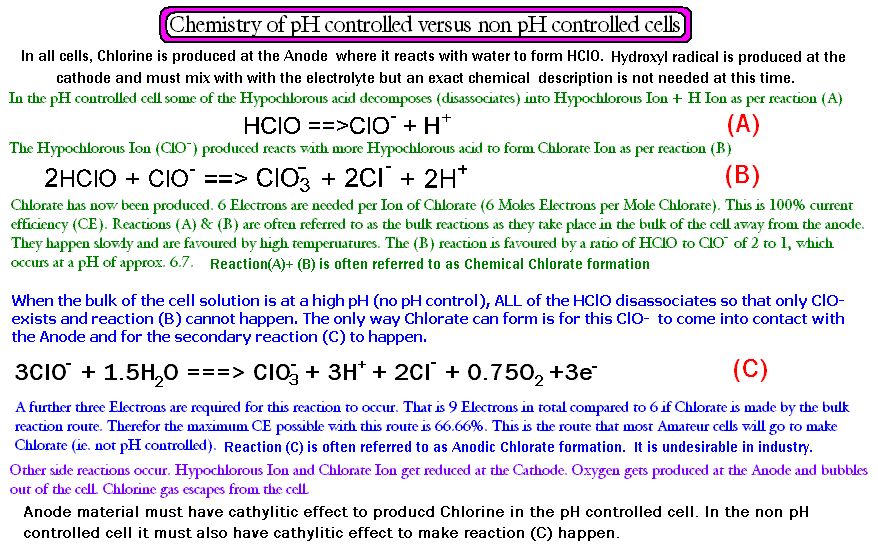
I think you will agree that the above explanation of cell chemistry is an exquisitely clear and terse explanation of what is going on   : : ..........perhaps not! ..........perhaps not!
The picture below explaines it better, I think.
The how, why and where of reactions A, B and C are really all you need to know about, in order to understand the vast majority (from our simple point
of view anyways) of what is going on in the cell. (you may also like to look at the graph of distribution of species (HOCl & ClO-) in the cell at
different (bulk of solution rem.) pH values, next post)
Dann2
[Edited on 6-11-2008 by dann2]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
This diagram just about sums up what pH controll is all about.
perhaps straying off topic a bit but what the heck.
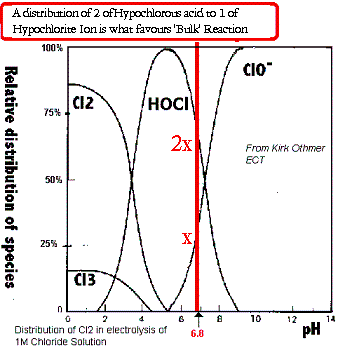
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Dann2, before I absorb your last post, I want to say - WOW - you saved my ass. As I mentioned before, the pump turnover is exquisitely slow, the EC
cell contents probably swap out only every 2 to 3 hours or so. I was adding, adding, adding HCl to the collection vat, which gets trickled into the
EC. I noticed that the EC pH was in fact coming down, around 7.5, but I wanted to check the vat pH as you suggested.
It was around 3! Way too low. The highly acidic vat contents were being injected into the EC, keeping that around 7.8 to 8.2 most of the time.
I turned the pump way up in an attempt to stabilize the system. Hopefully it'll calm down around 6 or 7.
Now I know why all my HCl dosing wasn't showing up. I was measuring in the wrong place! I probably administered 150 ml concentrated HCl into this 25
liter system in the last 30 hours, far more than my previous calculations showed was needed, probably 2X as much.
Would it be best just to let the system recover on it's own, or should I add a bit of NaOH? I don't want to screw up the reaction. I think I'll add
just a touch of NaOH and remeasure. I should be using KOH, but NaOH is all I have.
Reading your last post, it does make sense and is enlightening. My best efforts earlier yielded 60% efficiency, which is in-line with a non pH
controlled system. What I've learned in the last 24 hours will help. I think, even without a true continuous pH controller, I'll be capable of
maintaining pH between 5.5 and 7.5 with little trouble, perhaps even better. I just needed to learn where tto measure!
I hope I didn't screw things up too badly.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
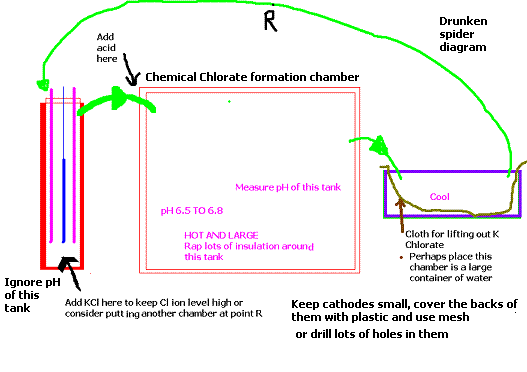
Looking at the diagram below, I think it is fair to assume that I would not cut it as a pilot. Moving swiftly along..................
Good to know there was a easily found problem.
Consider using your crystallization chamber as a 'chemical Chlorate formation' chamber. You will then have to add another chamber. I reckon your
electrode chamber is a bit too small (in relation to electrode size).
That's not to say this device will not work. You may get CE that are high enough, and it may not be worth the extra effort. The crystallization chambe
could be a bucket with a cloth in it just to see how things went. There would be litttle need for venting etc.
You could also consider drilling lots of holes in the cathodes to reduce surface area and putting plastic on the back sides of them where CD is
probably very low. (will stop/minimize cathode reduction).
Regarding your system at the moment I would be inclined to meter in what ever acid you think is needed and leave for 24 hours. (if your pumping system
allows that).
Why do you want to lower Chloride concentration down to 50 gpl or less? If you want to keep CE high you should keep it at 100 gpl or more.
MMO always seems to give good CE. You should be able to get 80 or 90% with pH control.
It is hard to give a figure on what the circulation rate of the whole system should be. I would guess if you pump the contents of the electrode cell
once every hour. (Wild assed guess).
Keep it up!!
Dann2
[Edited on 6-11-2008 by dann2]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Why not change the pH after cooling? I always added HCl until a bit too much chlorine was produced, after crystallizing the NaClO3.
Actively controlling pH (by rate of HCl addition or by actively monitoring pH) at the cell seems like a better idea to me, as its pH will gradually
rise no matter what. Adding HCl later sounds half assed.
Tim
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Something I have been thinking may be worth considering is closing the loop for recombinant chlorine as HCl recycle.
A small H2O electrolyzer of slightly excess of needed capacity could supply a steady stream of H2 into a reaction chamber iluminated by a UV lamp and
into which
the venting chlorine from the main cell is also supplied,
maybe something like a graham coil condenser for a tube reactor where the H2 and Cl2 are mixed and reacted to form HCl, and a small sprayer nozzle
would disperse some small diverted flow of the electrolyte from the electrolyte
recirculation loop , which would absorb the HCl and discharge back into the main cell. Such a scheme would
tend to keep the pH constant without attention or constant monitoring, by virtue of returning any evolved
Cl as HCl , closing the loop.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
That seems like it would be very difficult to do, Possibly Impractical for how much is being produce, as well as how cheap acids are. How would you
get it so the Cl2 + H2 can combine with out destroying whatever device it is in?
Also, Isn't a significant amount of H2 produced compared to the Cl2 that is released? You'd need to either let that off, or react that with Air and
capture it.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by kclo4
That seems like it would be very difficult to do, Possibly Impractical for how much is being produce, as well as how cheap acids are. How would you
get it so the Cl2 + H2 can combine with out destroying whatever device it is in?
Also, Isn't a significant amount of H2 produced compared to the Cl2 that is released? You'd need to either let that off, or react that with Air and
capture it. |
Glassware and teflon tubing I have aplenty. A glass graham condenser with a three way adapter on top and a small
spray nozzle for the electrolyte entering the center of the adapter , a UV rich (halogen) lamp shining on the gases mix coming in through a two hole
fitting on the sidearm of the three-way adapter, and the bottom discharge opening as return to the main cell or electrolyte reservoir. H2 and Cl2
combine spontaneously in the presence of light and the HCl
produced would immediately be absorbed by the aqueous
electrolyte as the mixture spiraled down through the glass
coil of the condenser.
Something like this would be far less expensive than pH monitoring and regulation by other means ..I think. If a cell is already using a pumped
electrolyte and a coaxial electrode,
along with a reservoir tank , this would be fairly simple to implement and it has no additional moving parts or sensors.
Anyway for a high capacity cell that is what I would do.
[Edited on 5-11-2008 by Rosco Bodine]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
| Quote: | Originally posted by Rosco Bodine
Something I have been thinking may be worth considering is closing the loop for recombinant chlorine as HCl recycle.
A small H2O electrolyzer |
Why another bit of hardware? You've got a rather large H2O (and other stuff) electrolyzer going on just inches below. Toss in a mesh platinum filter
and be done with it. (Oooh, do they make platinum wool? That would be awesome.)
Tim
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I'll have to review this one to be certain ...
but I believe you are right
http://electrochem.cwru.edu/ed/encycl/art-b01-brine.htm
I see what you are saying is that there may already be a mix of H2 and Cl2 in exact proportion coming off the cell similar as "Brown's Gas" coming
off the electrolysis of H2O as 2 H2 + O2 ????
Possibly all one has to do is react the cell evolved gases in an empty headspace inside the cell or in a column above , equipped with a UV source and
a spray nozzle for electrolyte at the top to provide an HCl absorption / precipitation tower...yeah that should work ...good idea.
You could just use a "gurgle column" filled with Berl saddles or beads, broken cubes of automobile safety glass
mixed with glass beads, short pieces of broken or intact
pieces of glass tubing and rod, hit it with a UV lamp and
pump a bit of electrolyte in at the top to keep all the
"plates" in the column wet without flooding it , so it
would work as a counter current absorption tower.
Platinum on alumina or shards of porcelain bisque might work, even scrap catalytic converter pieces might do the
trick as a column packing even better. I'm not sure and
would have to see what is specifically catalytic for HCl formation from the elements. Palladium on charcoal chunks
maybe ?
Interesting resource links, don't recall having seen this one
http://electrochem.cwru.edu/estir/
http://electrochem.cwru.edu/ed/encycl/art-e04-echem-soc.htm
concerning the photocatalytic reaction of hydrogen and chlorine
http://www.chem.leeds.ac.uk/delights/texts/expt_28.html
[Edited on 6-11-2008 by Rosco Bodine]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Before I can digest the evening input, I have a small situation to take care of...  Why does bad stuff always happen when you are asleep? Why does bad stuff always happen when you are asleep?
I went to bed with everything looking good. This morning, the DT (Temp Diff) between the two had gone from 30 to 60. The temp of the EC was 90
Celsius! I found out why...
When I built the thing, I created two ports on the big vat, one at 1/3 height, the other at 2/3 height. I plugged the pump input into the 1/3 height
port... which jammed solid with crystals in the evening chill. The EC flow ceased, and at 50 amps, the temperature soared. Hopefully no damage was
done. I dialed the current down to 2 amp just to keep the electrodes charged. It's cooling right now.
Two things I did wrong. I placed the pump inlet source too low on the collection vat, and I selected tubing with way too small a bore for the pump.
AND I should have installed some sort of prefilter for the pump. I'm lucky the pump didn't chew through the tubing and spring a leak. I should have
known better on all of these issues.
I am convinced this concept is workable. Everything was going as planned with the exception of the pH. I am going to tear down what I have this
morning, harvest what crystals exist, replumb, and fire it back up. It's going to be a noxious task.
My HCl dosing pump came with a beautiful mesh PVDF prefilter which I am going to cannibalize and use to protect the system pump; AND I'm going to plug
into the port at 2/3 height. There's no way crystals are going to reach that port.
When I'm done with this chore, I'll come back and read the latest inputs. But it's going to be a busy morning! 
[Edited on 6-11-2008 by Swede]
[Edited on 6-11-2008 by Swede]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Well, I've got some good news, plenty of bad news. Here's what happened...
The line leading to the pump was JAMMED with fine crystals. The crystals had not reached the port level in the collection vat; the liquor had simply
cooled, and once the solution was saturated with chlorate, fine seed crystals formed in the tubing, and from there, they grew.
With the pump in a no-flow state, the temperature in the EC soared. I measured 90 degrees C in the thermowell!

I took it outside and opened it up. The bottom had a layer of very fine crystals about 2" deep. Good news: the materials (CPVC and PVDF) held up
perfectly, which is remarkeable given those temperatures. The bad news - the cathodes. I have never sen anything like it, and I am at an utter loss.
They are both warped HUGELY in a symmetrical pattern away from the anode, which looked fine.
Check this out:


What the f***? All I can think of is that perhaps the TIG welds set up some sort of stress, which was released by the current? More bad news - the
cathodes are permanently installed, and the lid is ruined unless I don't mind warped cathodes. Really, really bizarre.
Continued; next post
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Hmm... interesting...
Hydride implanation? Hydride is lower density = expands on the near face (which carries most of the current). It's not weld stress, because the
curvature is along the length of the plate.
Heat it to release the stress and hydrogen. I suppose red hot would do it, which isn't a problem that far from the lid (titanium conducts poorly).
Though do keep a wet rag on hand just in case. Probably worth wet-ragging the anode too, so it doesn't get toasted in the process.
Tim
[Edited on 11-6-2008 by 12AX7]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I wheeled the collection vat outside... the cart IS the way to go for a heavy setup. I attempted to drain it from the side ports, but they too were
clogged hopelessly.
I popped the lid. There was a small amount of chlorate in the bottom, some nice, fat crystals, but not much. I'm estimating the chloride level in
the liquor went from 145 g/l to no less than 130, to deliver such a paltry yield. I expected this. the rig had not been running long enough for a
big yield.

With the exception of the clogged ports, everything was perfect. The lid, with all it's vent ports and gadgets, was likewise good.


I had installed those CP Ti cathodes earlier. I had a hunch that something catastrophic might happen, and I want to be able to run the vat as a
stand-alone cell. That's probably what's going to happen now.
I was forced to scoop out much of the liquor by hand, with a cup, to salvage what little chlorate there was.
Thoughts: Everything was going perfectly so long as the entire system was liquid. Sodium chlorate would not have given me this problem. Someone
needs to talk me into using sodium salts.
I was surprised at the amount of crystals formed in the EC. BUT, and that's a big but, I suspect everything I saw in there happened AFTER the
circulation stopped. I had 50 amps running in a relatively small cell, and it simply brute-forced a certain yield. If the liquor had been
circulating, I would have expected crystallization in the big vat.
I'm not sure this concept is sound with potassium salts. If it can be done, it'll require careful monitoring of the pump lines, which need to be
fairly wide-bore, and screening of the pump inlet. A thermostat needs to be set up to remove power if the temperature rises too high in the EC, which
would indicate a stopped pump.
A pump capable of carrying a slurry, and the use of hard CPVC pipe instead of tubing might also help.
For the moment, I am going to shelve the EC, and execute a carefully-controlled run with just the collection vat. With my old, crude setup, I was
getting a kilogram of fat, clean potassium chlorate crystals, and this vat is at least 4X the size. With no external plumbing beyond venting, the
system is dead-simple.
I'm down but not out. I'll find a way to boost efficiency. And the big question, what in the HELL hapened to my cathodes? 
No biggie. It's part of the research process. And Tentacles, you'll be happy to know that anode material looks like a winner for chlorate, so far.
[Edited on 6-11-2008 by Swede]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Swede: Perhaps the answer is to spot weld the cathodes, or reinforce them somehow. Maybe even pre-hydride them? A couple hours in a weak H2SO4
solution hydriding (use SS anodes) might make the stress more even. The strange thing is that when I've heard of the hydride warping phenomenon, it's
been said to take at least a year to become a significant problem. I think it's more likely a combination of weld stress and hydride warping, maybe.
I'm looking to get my hands on a MOT to make a spot welder soon. What kind of clamps did you use for yours?
At least the anode material works well! Maybe you should try it with sodium salt for a run, just to try the system as designed? Not like you couldn't
reassemble and try again later, but it could be fun. Think of the yield! HUGE! The problem, of course, is washing out the Na, but it's probably not
all that difficult once you've got perchlorate.
|
|
|
| Pages:
1
..
6
7
8
9
10
..
48 |