| Pages:
1
..
6
7
8
9
10 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Hennig Brand  | It still seems logical that having the reducing agent being made up mostly of sodium hydrosulfide would be best. If you look at the reaction equation
where sodium sulfide is used there is a mole of sodium hydroxide formed for every mole of sodium sulfide consumed. This seems to be the reason that
sodium sulfide, when used by it, results in such low yields. If sodium hydrosulfide is used as the reducing agent, the amount of sodium hydroxide in
the reaction mixture will be kept very low since the sodium hydrosulfide (acid salt) will neutralize, or mop up, the sodium hydroxide produced by the
reaction and form sodium sulfide in the process.
Still a little confused as to why sodium sulfide mixed with sodium hydrosulfide would actually produce higher yields than using just sodium
hydrosulfide by itself.
Reaction equation taken from Chinese explosives presentation slides attached. |
If you want to reproduce the method of Clayton as described in the Communications article then your yield should be 90% same as Clayton.
However, IMO if you want to try to increase the yield beyond that 90% of Clayton to the quantitative range described by Hodgson and Ward using an
equilibrium mixture, then you need to emulate that mixture by not taking to completion the formation of NaHS from Na2S via gassing with H2S, so that
some higher pH condition is retained by the mixture of Na2S and NaHS. Clearly the pH of the mixed Na2S and NaHS reagent of Hodgson and Ward is more
alkaline than the single NaHS reagent of Clayton. So there is the one most likely variable which accounts for the different yields.
See my additional general Zinin reaction references on the preceding page because our last posts have crossed in flight here, and I edited and added
references to my earlier post at the same time you were replying to the not yet edited earlier post.
[Edited on 4-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
That is a good find. Ok, I feel as though things are making more sense. I am on board with the sulfide ion being a better reductant than the
hydrosulfide ion. Obviously the Zinin approach of constantly monitoring pH and adding acid to keep the reaction mixture neutral is best, but the lab
method of Hodgson and Ward where they add sodium bicarbonate would get you part way there. It is a buffer, which would keep the reaction mixture
slightly basic, maintaining the equilibrium towards the sulfide ion side of things as well as tying up the sodium hydroxide produced in the reaction
and lessening its harmful effects (by-product formation).
NaHCO3 + NaOH → Na2CO3 + H2O
A sodium bicarbonate-sodium carbonate buffer solutions table is attached below, courtesy of Sigma Aldrich.
Something I just noticed in that Zinin article, " In some cases, where the presence of excess alkali is harmful, sodium sulfide is used with the
addition of magnesium salts which remove the sodium hydroxide as it is formed by precipitation of magnesium hydroxide". Sounds very interesting
indeed.
I took a few screen shots from a book on rubber chemicals, which describes production processes for sodium hydrosulfide and sodium sulfide.
Apparently, reacting hydrogen sulfide with a sodium hydroxide solution is the most common modern industrial process for sodium hydrosulfide and sodium
sulfide production. According to the text sodium hydrosulfide is prepared first and then, if desired, a measured amount of sodium hydroxide based on
stoichiometry is added to convert the hydrosulfide to sulfide.
NaHS + NaOH → Na2S + H2O
I know we are past this, but it seemed like a decent reference. I also think I would follow their lead and produce sodium hydrosulfide, using an
excess of hydrogen sulfide, and then convert some to sodium sulfide if desired. I don't think I would want to try and weigh the cylinder containing
the absorbent solution while the apparatus is in operation. Having the tubing and bubbler in place complicates weighing; even without the poison
danger it is a difficult situation. I think the best idea is to stay well away and use an excess of hydrogen sulfide and then add sodium hydroxide.
The solution could be weighed at the end though to verify its composition.
Attachment: Sodium Sulfide and Sodium Hydrosulfide Production - From the Complete Book on Rubber Chemicals.pdf (301kB)
This file has been downloaded 981 times
Attachment: Sodium Bicarbonate-Sodium Carbonate Buffer Solutions.pdf (130kB)
This file has been downloaded 4563 times
[Edited on 5-4-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The hydrosulfide ion works fine as the principal reducing agent but if used alone appears to be yield limited to 90% unless added methanol is the
trick that would account for increasing the yield. However it is just my guess really that pH is what is having the effect and is allowing for a
secondary and tertiary reaction system involvement when the reaction system is more basic. If you look at the 3 possible Zinin mechanisms, then you
can see that any free sulfur byproduct from the reduction by the hydrosulfide ion would react immediately with any normal sulfide present to form
sodium disulfide Na2S + S ---> Na2S2 and then
the Na2S2 would subsequently contribute to the reduction as well, but that mechanism would then have its byproduct oxidized sulfur tied up as
thiosulfate, similarly as would occur for the byproduct oxidized sulfur of the normal sulfide.
By using a mixture containing the normal sulfide along with the acid sulfide, you are enabling all 3 possible Zinin mechanisms to be occurring, in a
mixture where there is sufficient Na2S present that any free sulfur byproduct from the hydrosulfide ion mechanism to be recycled through formation of
soluble disulfide instead of accumulating as an insoluble precipitate or impurity in the desired product. By having some Na2S present it assures that
the sulfide oxidation byproduct from the reduction will occur as soluble thiosulfate or as unreacted soluble Na2S2 at the completion of the reaction,
instead of having a cloudy sulfur milk of precipitated free sulfur byproduct as an undesired contaminant for the sodium picramate. The higher
reaction kinetic value for the Na2S2 is likely what increases the yield beyond the 90% which is obtained using hydrosulfide alone as the reducing
agent.
The article posted earlier, Reduction of p-nitrotoluene by aqueous ammonium sulfide provides a good insight into the 3 mechanisms which are involved
and partly interdependent and sequential, having differing kinetics and occurring at different rates.
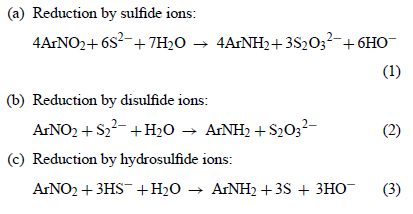
Therefore, for a mass reaction as would proceed from a mixing all at once of the reactants "in a lump" addition poured together to react, there is
going to be a most favorable proportional mixture of NaHS and Na2S that should give the highest yield. Some mathematical prediction of the likely
composition for such a mixture can be done, but the fine tuning of the mixture to identify the precise optimum ratio of acid sulfide to normal sulfide
could be done by experiment.
[Edited on 5-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I see what you mean about the elemental sulfur by-product when pure sodium hydrosulfide is used as the reducing agent. Also, I think I will save the
methanol and use water instead for my next experiment. Between us, I think we may have most of the pieces needed for the efficient production and
usage of picramic acid and DDNP. Getting this DDNP thing ironed out has been a long time coming for the hobby community. (Stops to pat self on back)

[Edited on 5-4-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
If you look at the relative "power" of the different sulfides as a Zinin reducing agent the reducing power on a molar basis per mole of nitro compound
for one nitro to be reduced to amino, the ranking from lowest to highest is
Hydrosulfide (3 moles) > Sulfide (1.5 moles) > Disulfide (1 mole)
However it is clear that a pH favoring the "mass reaction" leading to quantitative yield may be obtained by a mixture of hydrosulfide and normal
sulfide. Looking at the ratio of hydrosulfide to normal sulfide as would be optimal for subsequent reaction of the "nascent sulfur" byproduct of the
hydrosulfide mechanism, an equal number of moles of the normal sulfide would be desired to be present.
If the normal sulfide was stipulated to be a necessary to be present but "spectator intermediate" for the overall reduction while all of the actual
reduction could be attributed to the hydrosulfide and the "recycling" of its byproduct sulfur as the Sodium Disulfide. The equivalents of Zinin
reagents for the actual reduction of 4 moles of sodium picrate would then be 3 moles of NaHS plus 3 moles of Na2S (spectator intermediate) converted
to 3 moles Na2S2.
Based upon this admittedly oversimplified "projection" of a simplest terms most basic model for the reaction, an equimolar Na2S / NaHS reducing agent
would be the starting point with a minimum .75 moles of each to be used as the combined sum per each 1 mole of sodium picrate.
In actual reality the Na2S is not going to function as an idle "spectator" reagent simply waiting for free sulfur to be added to itself so it can form
Na2S2. A portion of the Na2S will proceed to act as the Zinin reagent it is, so that portion of the Na2S reacting unmodified and unconverted to the
disulfide will be unavailable to sequester the potential amount of free sulfur byproduct from the hydrosulfide mechanism. Therefore the optimum ratio
of Na2S to NaHS will theoretically be needed to be enriched aboved equimolar proportion with respect to the NaHS. A guess would perhaps be an
increase of one third to two thirds more than the amount of theory required. This estimate would then put the ratio of Na2S at 1 to 1.25 moles per
.75 moles of NaHS for each 1 mole of sodium picrate. If I were doing this experimental workup I would probably use a global 105% multiplier on the
quantities of reducing sulfur compounds in that range as a starting point for "excess of theory" of the reducing agent to "push" the reduction but
gently and allow for some loss compensation for air exposure during transfers.
As you observed before the NaHCO3 being present as a buffer would tend to keep the alkalinity limited to a milder pH associated with Na2CO3 by
reacting with the byproduct NaOH. I think the quantity of NaHCO3 is not especially critical so long as there is sufficient amount present, and the
quantity used by Hodgson and Ward would be good starting point, or that amount adjusted downward somewhat based upon the quantity of NaHS that would
be produced by gassing instead of produced in situ in methanol via the Hodgson scheme of conversion of the Na2S using NaHCO3.
Based on the admittedly inexact modeling described, I think a reasonable expectation for the yields should be at least somewhere in between the 90%
yields reported by Clayton and the near quantitative yields of Hodgson, so splitting the difference I think you could reasonably expect 95% yields.
Doing the calculations for the amount of needed NaOH to be gassed with H2S to achieve what weight is needed is all that is left for the theoretical
model.
[Edited on 5-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
So are you getting your glassware all dusted off for a series of experiments? 
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
No, but I wish my other priority chores were out of the way so I could do the experiments. One aspect about experiments I like is that things that
have been seen cannot be unseen. The Zinin reaction is complex and has never been truly identified as a single mechanism but can be 3 or more
concurrent reactions, and I believe there are likely 4th and 5th reactions that add to the list that would be Na2S3 and N2S4 and I don't believe the 3
finite mechanisms identified are absolutely governing, but are generalizations of what are predominating reaction mechanisms. For example when a "dry
process" reagent is made and modified in whatever subsequent ways that affect the sulfur rankings of the mix of reducing compounds that results, it
would seem that a mixed system of various polysulfides and sodium sulfide results which can still be a useful Zinin reagent as a mixed system that
defies analysis except according to inexact modeling as applies to the Zinin reduction itself. On a mathematical basis trying to identify with any
precision what is the exact composition of a polysulfides mixture and how that mixture accomplishes a reduction is an abstract algebra that compares
with trying to nail jello to a tree. Zinin reactions being explored comes down to experiments that are based upon some murky mathematics and end up
being a whole lot of "try this and see what results" 
For experimental results we have 3 possibilities:
[A] Yeah it worked just like I figured
[B] Well it worked on paper
[C] WTF!
And of course there may be a combined experiment result of indefinite proportions of the above three, or a not yet identified alphabet soup of other
possibilities which follows.
Rubicks cube and the Zinin reaction have commonality as hair pullers, yet there are solutions where everything seems to line up and the jello does get
nailed to the tree. A quantitative yield is that point, but the exact reactions are likely to remain enigmatic. So there is an appreciation of the
difficulty of Hodgson and Ward giving precise explanation of what was actually occuring in their own reported experiment, understanding that they
didn't really know 
It is almost a tongue in cheek academic joke when a high yield Zinin reduction is reported, to with great interest query the reporter as to exactly
what mechanism they attribute their results, knowing they are being assigned a perplexity.
Zinin likely understood the abstract mechanism aspect himself and probably derived great satisfaction from publishing a puzzler that would still be
puzzling a hundred years and more later.
This is a convoluted description, but I will try to explain generally how it is that even though following the method of Clayton, it is unlikely that
the actual reducing reagent predominating throughout the reduction will be the NaHS that is the starting material.
The potential variant reactions possible in a Zinin reaction system can become complex. For an example, take the third reaction mechanism of
hydrosulfide and see that the byproduct of 3 S and 3 NaOH could subsequently react to form an indefinite mixture of Na2S3 + NaOH and IMO it likely
does react further since the initial "nascent sulfur" byproduct would be highly reactive and would further react with whatever it could. And the
Na2S3 + NaOH as only one example could react with unreacted NaHS present, such as the following reaction/s:
Products of mechanism 3 are 3 S and 3 NaOH from the 3 NaHS used to reduce 1 mole of sodium picrate
The NaOH will react instantly with unreacted NaHS to form Na2S which will further react with the "nascent sulfur" to form Na2S2 and the Na2S2 could
also react with more "nascent sulfur" to form NaS3 and possibly Na2S4
(3) NaOH + (3) NaHS <-------> (3) Na2S + (3) H2O ( + 3 S ) -----> (3) Na2S2
According to this scheme then one half of the NaHS reacting according to mechanism 3 would convert in situ to Na2S2 the remaining half of the NaHS for
that mechanism as a result of the byproduct NaOH causing conversion of the unreacted NaHS to Na2S and reacting with free sulfur to form Na2S2. So it
is clear that even though you may start a reduction using only NaHS alone as the Zinin reagent, because of the complex subsequent chemistry involved,
when only the first half of that NaHS has reacted, the remaining half is already converted to other higher sulfides that are not NaHS but
mathematically according to theory would be Na2S2.
The Na2S2 formed is not a loss of reducing power for the second half-reaction of what was the original NaHS but is a multiplier of 3X the reducing
power of the first half of the NaHS consumed. So the overall reduction becomes one where half of the NaHS is consumed accomplishing only one quarter
of the overall reduction, while the remaining three quarters of the reduction are accomplished by conversion of the unreacted half of the NaHS
reacting with decomposition byproducts of the first reacting half of the NaHS.
If the Na2S2 does not react in the reduction immediately,
then it can itself react with the nascent sulfur byproduct
and it can further react with other materials present as follows:
Na2S2 + S -------> Na2S3 However the Na2S3 can further react also and be converted back to Na2S2
Na2S3 + NaOH + NaHS ----> 2 Na2S2 + H2O
The potential variant reactions like this soon makes it clear that a Zinin reaction is complex by its nature where the reaction system is in flux with
an indefinite mixture of reactions and subsequent reactions possible and probable.
I can guess that the reaction mechanism 3 is expected then
to provide a subsequent path to reaction mechanism 2 even for a reduction where the Zinin reagent being used for the reduction would be NaHS alone.
The reaction would likely not just neatly complete according to mechanism 3 but would "recycle" according to mechanism 2. So where does the free
sulfur byproduct come from? Obviously the "recycle" does not occur or does not occur completely, depending on the concentration and temperature and
pH. But I would bet good money that thiosulfate would be detectable in the completed reaction mixture, even for a reduction done completely according
to Clayton, bearing out what I describe.
How you can factor these variants into the estimated theoretical model is not yet nearly an exact science.
Way to go Zinin, nothing like keeping things simple, huh.
A Zinin reaction defies analysis in a way that is so artful that at the completion of the analysis and reaction modeling, the only firm conclusion is
likely to be that yes there is definitely sulfur involved in the reaction, just like I thought  I wasn't kidding when I said earlier that sulfur chemistry of this sort is a safari into the tall grass. I wasn't kidding when I said earlier that sulfur chemistry of this sort is a safari into the tall grass.
[Edited on 5-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
You were definitely right about the safari into the tall grass. I am definitely ready for some experiments; however I too have obligations (like
finishing the last few weeks of a degree). The theory is very complex; too complex in a lot of ways, but I understand the general principles a lot
better now and I think it will really help when it comes to running the next set of experiments. Getting those higher yields will also likely mean
higher purity right from the post reaction mixture. The low yields are often even worse than they would first appear, by looking at the scales,
because of the large proportion of bi-product contamination.
I am curious how much of this was review for you from things researched long ago and how much was new material. You seemed to locate and move through
this material rather efficiently.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Most of what we are discussing recently is review from files already saved years ago. Because of the ambiguous and complex nature of the Zinin
reaction and what sulfur contamination I think is likely in the product, I was intrigued by the alternate methods and was looking at other reductions.
But the high yields possible with the Zinin reaction has still intrigued me. A difficulty inherent with calculations for performing experimental
Zinin reductions is the complexity of the possible reactions involved. The literature trends towards oversimplified descriptions of what is a complex
reaction that isn't as simple as it may appear in a summary equation from a reported synthesis. But the reported good yields for certain Zinin
reactions certainly makes them interesting for that reason, while the reaction mechanism is a classic academic curiosity also.
The mathematics of the reaction calculations of Hodgson and Ward is something that would need to be checked again and revisited with analysis
reviewing with new comprehension the calculational discrepancy which was appearing before, in light of the recent discussion of the "recycle" type of
reaction occurring for an NaHS reducing agent.
As an example, suppose 2 moles of sodium picrate was planned to be reduced by NaHS believed to be the reductant which would be consumed 100% according
to mechanism 3.
6 moles of NaHS would be required by "simple theory" to balance that equation represented only as mechanism 3. Yet an unexpected development has
occurred when 3 moles of NaHS have reacted, the other 3 moles of the NaHS have "disappeared" through reaction with the byproducts, but not at all
disappered from the continuing reduction because the newly formed 3 moles of Na2S2 into which the "missing" 3 moles of NaHS have been transformed
acquire a tripling of the former reducing ability. What was originally the 3 moles of remaining NaHS available for reduction of the remaining 1 mole
of sodium picrate, has become 3 moles of Na2S2 capable of reducing 3 moles of sodium picrate, while only 1 mole of sodium picrate remains.
So we see the actual "reducing power" of the NaHS originally calculated only according to mechanism 3 has been upgraded in situ by the midpoint of
that oversimplified reaction mechanism 3, so that overall the actual reducing power of the originally provided NaHS is doubled, as a result of the
appearance of mechanism 2 for half the NaHS that is present. By that development the same 6 moles of NaHS as first thought to be needed to balance
the equation and reduce 2 moles of sodium picrate, has become sufficient to reduce 4 moles of sodium picrate. So the original reaction model based on
NaHS reducing power according to mechanism 3 is an incorrect calculation based on neglecting to consider the subsequent "recycle" into mechanism 2 and
results in attributing to NaHS only half of the actual reducing power
that it actually has, when the "recycle" into mechanism 2 is factored into the model.
Hodgson and Ward may have revealed that they are aware of this complexity about the Zinin reaction, by what may first appear to be an error in
stoichiometry, but which is actually not an error. So the stated quantities would need to be interpreted using the multiplier that accounts for the
involvement of the mechanism 2 pathway, which effectively doubles the reducing power of NaHS estimated by mechanism 3 alone, and results in only half
the quantity of NaHS that would be required according to theory for that mechanism alone. I think I had looked at this possibility
years ago for the Hodgson and Ward article but don't recall what was my conclusion then, but IIRC it did explain what first appeared to be a
discrepancy about their numbers.
I think in reviewing this recently we were talking in a circle around this discrepancy that is seen in the Hodgson and Ward article, but there is no
simple way to describe this aspect of the Zinin reaction, and no textbooks that I have ever seen are very helpful about revealing this "nuanced" and
broader interpretation of the actual Zinin mechanisms, with a good breakdown and description of the 3 reaction mechanisms as a "strange brew" of
complex reactions proceeding in parallel.
My impresssion of performing a Zinin reaction is something like churning a sulfide reeking "milk of sulfur" deadly brew while waiting to separate the
cream that is the product, and I still call it a *nasty*, potentially deadly reaction. The hydrolysis of inherently hydrolytically unstable Zinin
reagents and products is always producing some H2S and it is something comparable to working with a little cyanide, not a really user friendly
proposition. H2S is deadly toxic and nobody should ever become complacent about it.
<iframe sandbox width="640" height="480" src="//www.youtube.com/embed/oElnOb_ookE?rel=0" frameborder="0" allowfullscreen></iframe>
[Edited on 6-4-2014 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
A deeper look into the Zinin reaction
There is some more analysis I have done in reviewing this topic and looking at my old notes. Mower time for the tall grass. To analyze this reaction
further and avoid a lot of fractional odd number molar quantities that are harder to follow for visualizing these molecule parts is easier with whole
lego blocks, so there is applied an algebraic multiplier across the reactions so that the stoichiometry comes out as whole number values easier for me
to then inventory the results for these parallel mechanisms for the range determination of NaHS required by theory.
Based upon the earlier described process the Zinin reaction mechanism 3 can be added to the Zinin reaction mechanism 2 to better describe what is
occuring, taking into account the recycle of byproducts from reaction 3 continued as reaction 2.
Here is the summary diagram again for the three main Zinin reaction paths. My multiplied values in equations below this summary diagram will
correspond to these ratios as the same reactions stoichiometry held true but expanded by a multiplier to avoid fractional value results.
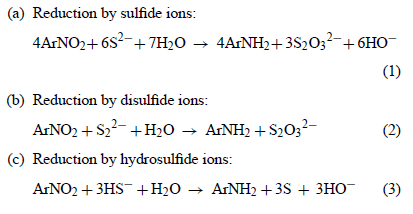
The summary equation resulting for reaction 3 with reaction 2
1 ArNO2 + 3 NaHS + H2O ----> ArNH2 + 3 S + 3 NaOH
3 NaHS + 3 S + 3 NaOH ----> 3 Na2S2 + 3 H2O
3 ArNO2 + 3 Na2S2 + 3 H2O ----> 3 ArNH2 + 3 Na2S2O3
4 ArNO2 + 6 NaHS + H2O -----> 4 ArNH2 + 3 Na2S2O3
If the reduction using NaHS as described by the Clayton Communications article proceeds according to the scheme above then for each 1 mole of sodium
picrate there will be needed 1.5 moles of NaHS
However, this 1.5 moles NaHS requirement takes into account only the parallel reactions of Zinin reaction mechanism 2 and reaction mechanism 3. But
it will also occur to unknown extent that a parallel reaction of mechanism 1 can occur. Formation of Na2S from the NaOH byproduct from reaction
mechanism 3 will be instantaneous. Na2S thereby formed can either further react with the free sulfur byproduct or in the alternative the Na2S can
react with the sodium picrate as reaction mechanism 1. While the earlier parallel reactions involving mechanism 3 and mechanism 2 would not greatly
change the pH of the reaction mixture, this reaction mechanism 1 can (and does) account for increasing alkalinity of the reaction mixture and may be
buffered by the presence of NaHCO3, moderating that rise in pH which is already identified by Hodgson and Ward to greatly reduce the yield.
4 ArNO2 + 6 Na2S + 7 H2O ---> 4 ArNH2 + 3 Na2S2O3 +
6 NaOH
This reaction mechanism produces the need for neutralization as is described in the fundamental processes of dye chemistry article.
If reaction mechanism 1 is considered to be an equal possibility for how the Na2S derived from reaction mechanism 3 may proceed, then perhaps half of
the Na2S will then act directly according to reaction mechanism 1, while the other half of the Na2S will react with free sulfur and form Na2S2 which
will proceed according to reaction mechanism 2.. The actual extent to which reaction mechanism 1 occurs is what will govern the elevation of pH and
need buffering or neutralization.
A different scaling multiplier should be used to arrive at whole number quantities.
Reaction mechanism 1 as in Clayton Communications article
4 ArNO2 + 12 NaHS + 4 H2O ----> 4 ArNH2 + 12 S + 12 NaOH
Subsequent reaction of decomposition products with remaining half of unreacted NaHS
[ 12 NaHS + 12 S + 12 NaOH ----> 12 Na2S2 + 12 H2O ]
( if 100% of Na2S reacts with the free sulfur )
In the alternative, if 50% of the Na2S further reacts with half the free sulfur
6 NaHS + 12 S + 6 NaOH ------> 6 Na2S2 + 6 H2O + 6 S
And the other 50% of the Na2S instantly forms and acts as a reducer
6 NaHS + 6 NaOH ------> 6 Na2S + 6 H2O
4 ArNO2 + 6 Na2S + 7 H2O ---> 4 ArNH2 + 3 Na2S2O3 + 6 NaOH
6 ArNO2 + 6 Na2S2 + 6 H2O ------> 6 ArNH2 + 6 Na2S2O3
14 ArNO2 + 24 NaHS + 4 H2O -----> 14 ArNH2 + 9 Na2S2O3 + 6 NaOH + 6 S
For each 1 mole of sodium picrate 1.7143 moles of NaHS would be required by theory for the reduction based upon the proposition that 50% of the Na2S
forming in situ from self-reaction of the NaHS decomposition byproducts then proceeded to act directly as a reducing agent, while the other 50% of the
Na2S first reacts with half of the free sulfur to form Na2S2 and then proceeds to act as reducing agent. Ideally and most desirably it would be
wished that 100% of the instantly formed Na2S would await reacting with the free sulfur and form Na2S2 which would then reduce the sodium picrate
without having any affect on the pH since the only byproduct of that mechanism is sodium thiosulfate. But we know that some free sulfur is going to be
present which indicates that part of the Na2S is reacting differently and is otherwise occupied and does not form Na2S2. As a result the reaction
mixture moving in the direction of reduction is becoming more alkaline which tends to quench the reduction as that alkalinity increases. So the ideal
reaction where all of the Na2S would be converted to Na2S2 does not occur and this increases the amount of NaHS required for the reduction of 1 mole
of sodium picrate from the 1.5 mole of that ideal reaction to 1.7143 moles based upon a 50% conversion for the Na2S to Na2S2.
Next there may be examined the worst case scenario where 0% of the instantly formed Na2S reacts with free sulfur and instead proceeds to reduce the
sodium picrate. Calculations based upon the worst case scenario should then provide the upper limit for the amount of NaHS required by theory per 1
mole of sodium picrate, so that the range of NaHS possible to be a required minimum is then known. For the worst case
scenario there are 2 moles NaHS required by theory for each 1 mole sodium picrate. So for range from ideal reaction 1.5 moles to worst case scenario
2.0 moles or 1/3 more NaHS on a molar basis would be required by theory, is not much difference.
8 ArNO2 + 12 Na2S + 14 H2O -----> 8 ArNH2 + 6 Na2S2O3 + 12 NaOH
Materials possibly useful as a buffer is an ammonium chloride / ammonia solution, or perhaps an ammonium chloride / ammonium acetate mixture.
My impression from observing the reaction done using the more crudely improvised polysulfide containing dry method reagent is that the reduction runs
closer to ideal than to the worst case scenario based upon the amount of free sulfur not being great but seeming to be a nuisance contaminant, not a
large amount being produced as a reaction byproduct, more like a significant but minor amount trace contaminant.
Reviewing the Hodgson and Ward article again it appears that for reagent (A) that it is indeed 4.6 moles of reducing agent used for reducing agent (A)
and that it is an equimolar mixture of Na2S and NaHS and NaHCO3. Reducing agent (B) is Clayton’s reagent. A caveat of sorts is described in the
Hodgson and Ward article where they describe testing the reaction mixture to determine when free sulfide was accumulating and showed the end of the
reduction. Obviously a positive test would have caused or should have caused interruption of the addition of that reducing agent (A) when only half
of the quantity had been added, since half that amount described being made up as reagent (A) is what value is charted as 2.3 and this is where the
Hodgson and Ward article descrption becomes enigmatic. But regardless of Hodgson and Wards number discrepancy I am pretty sure my numbers and
analysis are correct. If the reducing agent (A) is an equimolar mixture of Na2S and NaHS as described, then that mixture could be achieved by
interrupting the H2S addition when the weight of the mixture showed that half of the initially formed Na2S has been converted to NaHS, and then the
same molar amount of NaHCO3 would be added as 1/3 the molar amount of the NaOH used to produce the sulfides mixture.
However, it may be that an alternate buffer scheme using ammonium salts could perform better than NaHCO3.
It seems that Hodgson and Ward were in the general area of examining the effect of ammonium salts but their approach was not correct, as if they did
not fully understand the Zinin reaction, which is understandable since no one does even after more than 150 years of chemists experimenting with it.
The kinetic studies look at the byproducts and try to perform a post mortem on the reductions to guess what mechanisms predominated and led to the
products found.
While doing this review there was attention renewed on a patent US2705187 which is classic process chemistry for useful reducing sulfur compounds
being produced efficiently by a non-H2S direct reaction of NaOH and S. The temperatures are within operating limits of fluoropolymers which would
seem workable as an alternative to expensive alloys. An FEP or PVDF or PFA lab bottle or cartridge filter housing in an oil bath may be workable as a
reactor. Im not sure that even the etching would be too excessive for some heavier ceramics or pyrex over the short term since the exposure time is
brief. Expendable glassware might be okay in a limited use for this. The etching could prepare it for later adherent coating with a hot melted film
of FEP or PFA.
One of the aspects of the reduction schemes using iron and iron salts that would be a beneficial feature is the more pH friendly byproduct Ferric
Oxide which doesn't leave a bumper crop of free radical hydroxide as a byproduct driving the reaction mixture alkaline. There are patent schemes
where ferrous salts are used along with alkali sulfides in a kind of hybrid reaction where it is likely pH control is more easily done. So it is
possible to combine reducing agents and schemes in the same pot and there may be advantage to those hybrid reducing schemes that are part Zinin and
part other. The cleanest reduction out of the portfolio of various methods possible is probably the reduction using zinc powder, and that method
would probably work similarly to the reduction of nitroguanidine to aminoquanidine. A few possibilities not mentioned are magnesium in methanol as
magnesium methoxide, Clemmensen Zinc, or aluminum amalgam. Alkaline sugar and ascorbic acid have been mentioned already. So there is plenty of room
left for experiments for this reduction.
[Edited on 8-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I said above that for my next experiments that I would save the methanol and go with water. This is apparently a bad idea if the reducer is sodium
sulfide and the sodium bicarbonate buffer is used. From Hodgson and Ward, "while the addition of sodium bicarbonate to the sodium sulphide in alcohol
greatly improved the reduction efficiency, this does not occur in water alone (see Table II, Experiment 6) since the water-soluble sodium carbonate
formed (insoluble in the methanol medium) probably introduces the alkalinity necessary for by-product formation with 20% diminution in yield of
picramic acid." Makes sense now that I stop and think about it.
I might try the following in water though. The idea, which came from the Zinin article, is to use a magnesium salt that would precipitate the
hydroxide formed from the reduction reaction, as magnesium hydroxide, thereby keeping the pH of the reaction solution from rising and preventing
undesirable by-product formation. Epsom salt is magnesium sulfate heptahydrate and would probably work fine.
MgSO4 (aq) + 2NaOH (aq) -------> Mg(OH)2 (s) + Na2SO4 (aq)
If it wasn't possible to separate the magnesium hydroxide and sodium picramate from each other after the reaction was complete, the sodium picramate
could be precipitated as usually and then dissolved in water before filtering out the magnesium hydroxide. Acidifying the sodium picramate solution
would then precipitate picramic acid. I think my next experiment may involve Epsom salt.
I am not sure how difficult it will be to remove the precipitated magnesium hydroxide from the sodium picramate solution. It seems like it may be
difficult to filter. As long as it can be removed with a reasonable amount of effort, this process would likely be a good way of reducing bi-product
formation and increasing yields.
[Edited on 9-4-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Looking at this now I am beginning to favor my original impression that the Hodgson and Ward description of reagent (A) is actually describing what I
first thought it was, which is simply Na2S + NaHCO3 + H2O which is what they are regarding as "equivalent to" when they say "containing" sodium
hydrosulfide 5.6g., 0.1 mole, NaHS. Hodgson and Ward reference Hodgson and Birtwell as precedent for reagent (A) and it is clear that Hodgson and
Birtwell are describing just Na2S + NaHCO3 + H2O. With the amount of H2O at 40ml plus 8ml included water from the Na2S - 9 H2O mixed with 8.4g NaHCO3
this would have to be kept warm to make solution in the first place and kept warm to prevent the solution from solidifying on cooling because of the
formation of the decahydrate of Na2CO3 would otherwise cause the mixture to set up solid on cooling.
For the Reagent (A) of Hodgson and Ward
Na2S + NaHCO3 -----> ( Na2CO3 + NaHS )
For table II, Experiment 7 to actually correspond accordingly would have required 10.6 g of Na2CO3 rather than the 2 g Na2CO3 that were used. The 80%
yield reported is less than the 90% yield reported by Clayton for the NaHS used alone. NaHCO3 would probably have better effect.
Another idea is that ethyl acetate could be useful for limiting the alkalinity via saponification to ethanol and sodium acetate. Ethyl acetate is
soluble in water to an extent of about 7%
CH3CO2C2H5 + NaOH → C2H5OH + CH3CO2Na
Dealing with the excess alkalinity from the Zinin reaction is something that I think is possible to be addressed with a hybrid method where only part
of the reduction is done by the Zinin and the alkalinity produced is managed by a ferrous or manganous salt sufficient to accomplish the remainder of
the reduction at the same time neutralizing the alkalinity. Ferrous Sulfate or Manganous Sulfate produces a hydroxide of that metal which has
reducing properties and is converted to the Ferric or Manganic oxide, while the alkalinity byproducts from the Zinin become the normal sodium sulfate,
or if the chlorides or acetates were used, then likewise the corresponding sodium salt is the byproduct. The soluble ferrous and manganic salts will
also precipitate and remove as filterable insoluble or low solubility byproducts unreacted sulfur and sulfides from the spent Zinin reaction.
[Edited on 10-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Yeah, I was thinking after I wrote the above post that maybe magnesium chloride might be a better choice than magnesium sulfate. Magnesium chloride
forms essentially neutral solutions and magnesium sulfate forms slightly acidic solutions. I wonder though if the amount of acidity produced from the
small amount of magnesium sulfate needed, in the rather large volume reaction mixture, would be much of a problem. The magnesium sulfate could be
added to the well stirred reaction mixture, incrementally during the course of the reaction as a solution, which may be preferable to adding it all at
once at the beginning of the reaction. Using a magnesium salt would be much safer than adding a strong acid like sulfuric acid to control the pH
because of the risk of producing hydrogen sulfide gas.
[Edited on 10-4-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It is actually the physical nature of the gelatinous hydroxide which would likely be a hinderance for magnesium which would make me not think that is
a good idea. In the alternative, to use manganous or ferrous salts, the hydroxide has three advantages, [1] the reducing ability of the ferrous
hydroxide reduces the quantity needed for the Zinin reagent and thereby reduces the byproduct sulfur load [2] the ferrous hydroxide leads to a ferric
oxide byproduct that is filterable and causes no remaining alkalinity but instead removes it by double decomposition [3] whatever free unreacted Zinin
reagent and byproduct sulfur is present will be sequestered by reaction with the iron or manganese as low solubility sulfides, with whatever portion
that is soluble also being able to act as a reducing agent but not having any byproduct alkalinity.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Looking in my bottle of milk of magnesia, it does indeed looks like it would be very difficult to filter. It would likely just plug up any normal
filter. I guess I will be doing methanol and sodium bicarbonate for my next experiment. I wonder if a flocculent could be used to get the magnesium
hydroxide to collect and settle.
Attached is an article regarding the solubilities of sodium bicarbonate and sodium carbonate in methanol-water mixtures and acetone-water mixtures.
Attachment: Solubilities of Sodium Carbonate and Sodium Bicarbonate in Acetone-Water and Methanol-Water Mixtures.pdf (190kB)
This file has been downloaded 1770 times
[Edited on 11-4-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Here are some patents of interest
US1689014
US1765660
US1878950
Attachment: US1689014 Reduction using Ferrous Sulfide from Ferrous Sulfate.pdf (214kB)
This file has been downloaded 863 times
Attachment: US1765660 Manganous Sulfide reduction of nitroaromatics.pdf (94kB)
This file has been downloaded 962 times
Attachment: US1878950 Sodium Polysulfide reduction of nitroaromatics.pdf (316kB)
This file has been downloaded 817 times
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rosco Bodine  | | Looking at this now I am beginning to favor my original impression that the Hodgson and Ward description of reagent (A) is actually describing what I
first thought it was, which is simply Na2S + NaHCO3 + H2O which is what they are regarding as "equivalent to" when they say "containing" sodium
hydrosulfide 5.6g., 0.1 mole, NaHS. Hodgson and Ward reference Hodgson and Birtwell as precedent for reagent (A) and it is clear that Hodgson and
Birtwell are describing just Na2S + NaHCO3 + H2O. |
Yes, you are correct in my opinion. I think that article was deliberately vague and ambiguous, especially given how well the author expressed himself
when he felt like it. Given everything I understand now, methanol, sodium bicarbonate and sodium sulfide is a great combination when used in the
correct proportions. I spent half the day experimenting; made more sodium sulfide and reduced 5g of picric acid. I am going to wait until tomorrow
until I am sure the product is really dry to report the yield, but it looks fantastic and the filtrate was a nice semitransparent sodium picramate
color, not the deep red/purple color that is usually seen when there is a lot of bi-product dye(s).
Regarding hydrogen sulfide generation, I figured out why my generator quit early the last time. The steel delivery tube (brake line) was completely
plugged. I used a propane torch and heated the tubing, burning away a lot of the blockage material (seemed to be mostly sulfur). It plugged again
though after about 20 minutes of H2S generation. I cut the power after the rubber stopper blew out (tighter fitting stopper this time) and
waited half an hour or so for the H2S to dissipate before retrieving the sulfide solution. I guess I need a bigger gas delivery tube, and a
better way of cleaning it also.
Eight grams of sodium hydroxide was used with water to make about a 75 mL solution. About 150% more sulfur and Vaseline than theoretically needed to
convert all the sodium hydroxide to sodium hydrosulfide was used in the generator , though of course it was not all used. The graduated cylinder was
weighed before absorption and after. Even though the generator only ran for 20 minutes or so, the weight of the solution had increased by about 3g.
This means that nearly all the sodium hydroxide had been converted to sodium sulfide. For simplicity the solution was treated as being composed only
of sodium sulfide. About 2 moles of reducer was used for every mole of picric acid.
Monoreduction of Picric Acid - Experimental
Reactants:
5g TNP
0.9g NaOH
3.7g NaHCO3
35mL aqueous sodium sulfide solution (described above)
Reaction Solvent:
50mL MeOH
water from sulfide solution
The 0.9g of NaOH was dissolved in 50mL of methanol in a beaker under magnetic stirring. Once dissolved, the 5g of picric acid was sprinkled in under
strong stirring. The 3.7g of NaHCO3 was dissolved in the 35mL aqueous sodium sulfide solution before being pipetted into the reaction flask
over a 3 or 4 minute period. There was a thick, yellow/orange precipitate, but the stirrer was not decoupled and kept going. About 2 to 4 minutes
after the last addition an induction point was reached and the temperature rose from 40oC to 60oC over the course of 2 minutes
and the reaction mixture turned dark red. The reaction was allowed to cool back down to 40oC or so over the course of 15 minutes under
strong magnetic stirring. After the temperature had dropped below 40oC an ice bath was added and the temperature brought down to
15oC before 75mL of ice water was added to aid precipitation of the sodium picramate. After 10-15 minutes, and with the reaction mixture at
about 10oC, the sodium picramate was separated from the mixture by gravity filtration. Yield looks very good.
I was unsure of how well this would work because the amount of water was too large really (about double what it should have been), also there was some
unconverted sodium hydroxide present. Looking at sodium bicarbonate and carbonate solubilities in methanol water mixtures, this extra water would
result in at least ten times the sodium carbonate being in solution if common ion effects on solubility are ignored. Some sodium carbonate in solution
is still a lot better than an equivalent (or more) amount of sodium hydroxide in solution though. If the gererator had not plugged, the 8g of sodium
hydroxide would have been converted to sodioum hydrosulfide which later could have been converted to sodium sulfide by adding another
4g of sodium hydroxide to the solution.
I will post the yield tomorrow, once it's dry.
    
[Edited on 11-4-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Excellent to have the Hodgson and Ward ambiguity sorted out. I had an idea further that the reaction of NaHCO3 and Na2S to form NaHS would occur
anyway in H2O even without being solubility driven towards completion by the insolubility of Na2CO3 in methanol. But the methanol could benefit the
reduction as solvent for some intermediate in the reduction and could be general, no matter what reducing agent is used, so that the methanol may have
value apart from any effect regarding Na2CO3 solubility. The presence of some Na2S already waiting to react with free sulfur byproduct that would
appear from the reaction mechanism 3 involving reduction by NaHS, would favor the formation of Na2S2 which is the most active of the Zinin reagents.
So it could be the reaction is being steered towards Na2S2 formation by use of that mixture of Na2S and NaHCO3 as described by Hodgson and Ward. If
the high yields which are being reported by Hodgson and Ward are confirmed then there is one good working method of synthesis identified and verified.
The subliming of sulfur at the high temperature needed for the production of H2S from 30 parts vaseline and 70 sulfur
is an issue reported in the literature for that method. Evidently half of the sulfur that theoretically could react to form H2S is instead sublimed as
a mixed vapor with the H2S and then the sublimed sulfur condenses on cooler portions of the flask or in a larger bore condenser or cold trap.
Attached is an article describing some older H2S generation schemes including the vaseline and sulfur reaction which is a pyrolytic cracking.
Also is linked a good page with some useful information about H2S generation.
http://sulphur.atomistry.com/hydrogen_sulphide.html
There is an alternative of wet methods for producing hydrogen sulfide from the mixture of sulfides and polysulfides from reaction of sulfur with
sodium hydroxide, possibly in mixture with calcium hydroxide if the mixture should prove more economical, then using HCl for the neutralization.
Relatively easily prepared indefinite mixtures of polysulfides contain a significant amount of combined sulfur which will be converted to H2S upon
acidification, although much of the sulfur will instead of conversion to H2S be precipitated as free sulfur. Sulfur is cheap and hydrated lime is
cheap and washing soda is cheap, so it would seem that a mixture being heated and stirred would form a mixture of sodium sulfides and polysulfides
with a precipitation of calcium carbonate which could be filtered out. The residual mixture on acidification should produce H2S.
Attachment: Hydrogen Sulfide Generator Pages from Proceedings_of_the_American_Pharmaceutic.pdf (304kB)
This file has been downloaded 774 times
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for the good information on hydrogen sulfide. I guess I wasn't using nearly as much of an excess as I thought. That webpage you linked to above
has some really good information about hydrogen sulfide.
Monreduction of Picric Acid - Yield
I finally got the sodium picramate from yesterdays experiment nice and dry. From 5.03g of picric acid 4.62g of sodium picramate was obtained, or about
a 95% yield. A small amount was not recovered from the filter paper and a small amount was lost during the post filtration wash with ice cold water,
but all in all a very good yield indeed. No I did not fudge the numbers, they are real. 
A picture of the dry yield is attached below.

[Edited on 12-4-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Really good results you have there, and the yield at 95% is about what I was guessing it should be. There are different schemes that can be used for
generation of H2S from various easily obtainable and cheap materials, so the general method is very adaptable to various schemes which may be more or
less convenient based on the scale and what raw materials are available. DDNP is the original "green energetic" initiator, so this is a good
historical interest material still having practical application, even though it does require using more weight of the DDNP as an initiator due to its
sluggish DDT which consumes as "kindling" a fair amount of the material during the runup accelleration from ignition progressing to high order
detonation. DDNP will still get the job done so long as enough is used being mindful of that DDT aspect and limitation.
[Edited on 12-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thanks, this is definitely more like it for sure. And, once again, thanks for your help. With regards to the initiating ability of DDNP, I have found
that as long as it is not over pressed and strong confinement is used it can initiate picric acid, to high order detonation, using
weights very similar to what is needed when lead azide is used.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yes weight for weight DDNP may be half as good as Pb(N3)2 but the same weight of DDNP requires a substantially larger ID cap to comfortably cram
enough of the much lower density DDNP in the detonator volume available to get the job done. DDNP is therefore disadvantaged by both its equivocality
for substantial DDT critical mass, and disadvantaged by also being a lower density material requiring additional volume to compensate for both
differences. Simultaneously the required adjustment of geometry will address the larger critical diameter for DDNP which is also in play. Usually
these properties are interdependent. It is the same story generally for any detonator design, the very small dimension detonators require very hot and
very fast high density unequivocal initiators like silver or lead azide to get the job done in a small dimension package, but using lower performance
materials can easily double the geometry needed. Even really low performance initiators can work if the detonator dimensions are scaled up enough for
accomodating the larger critical diameter and multigram initiator charges required for the high order velocity to be attained. For example a lead
styphnate initiator or lead picrate initiator type of detonator can be made but they are inconveniently large so they are not really as practical as a
more reasonably sized detonator using higher efficiency initiators. DDNP has better performance than those examples of what would be a really poor
initiator. So DDNP is fairly identified as a kind of intermediate performance initiator among the various choices of possible initiators. But the
literature seems to describe DDNP in more glowing terms and favorable review than would deservedly be a more conservative and practical review.
DDNP is workable, but the experimenter must rely upon their own test results and not rely upon what is described in much of the dubious "trusted
literature" which is simply not so trustworthy or experimentally verifiable in its claims for DDNP. Read the literature with healthy skepticism.
Then your own experimental results are less surprising when you see what is actually true.
I did an experiment and my experiment doesn't say what the book says. How about that.  The book is Wrrrrr....wrrrr...WRONG! The book is Wrrrrr....wrrrr...WRONG!  But your experiment is
right. But your experiment is
right.
[Edited on 12-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Well I will try to not get into one of those my primary is bigger than yours arguments, but anyway.   
My last experiment (before I ran out of reinforcing caps) involved the high order detonation of picric acid initiated with 0.4g of DDNP. The picric
acid was in a 7.6mm casing and the DDNP was in a 1/4" reinforcing cap placed within the same 7.6mm casing. It looked as though I may have been able to
drop the weight even lower than 0.4g, and still achieve high order detonation, but I haven't done the tests yet. Everything is based on some standard,
but as far as I am concerned DDNP is a high performance primary. For instance in comparison to TATP, DDNP is a very high powered primary. It doesn't
have the ability to detonate almost instantaneously unconfined like lead azide does, which makes it less convenient to use however. I think the
literature may be correct, and that DDNP may be superior to lead azide for initiating explosives like picric acid and TNT. Then again it depends on
how you define superior. In terms of ease of use and high tolerance for incorrect loading, etc, lead azide is far superior. I would go so far as to
say that DDNP requires 10X the education to use effectively as what is needed for lead azide. Lead azide is extremely forgiving of a lack of
understanding, with quite large departures from the ideal still producing acceptable results.
DDNP is much less dense than lead azide, which is true, but when you are using 2g picric acid base charges a little extra cap length isn't that big of
a deal , especially for the hobbyist. DDNP has very high sensitivity to flame, which is an advantage over lead azide. DDNP is also relatively
insensitive to most other forms of stimuli making it a relatively safe primary. Also as you pointed out, it is fairly "green"; it does not contain
heavy metals.
I think lead azide is better when used in small caps with more sensitive secondaries like PETN and RDX. I think DDNP can really hold its own when used
to initiate picric acid or TNT when used in a reinforced cap configuration.
I am going to have to try silver azide coated DDNP. Apparently a couple to a few percent of silver azide can be formed on the surface of other less
unequivocal primaries producing a primary that is much more unequivocal. Maybe that would solve a lot of the inconvenience with DDNP.
I think lead azide is a much more convenient primary for most applications. I also think that very few people have successfully made DDNP, and even
fewer people have a clue about how to effectively use DDNP.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
hydrated protosulphide of iron
Here is a bit of interesting information from antiquity. Everyone knows about the basic chemistry student experiment of heating iron filings and
sulfur to the ignition point to form ferrous sulfide. But here is a variation on that procedure which involves a still vigorous reaction which can be
moderated with water so that in the presence of steam generated in situ the reaction proceeds similarly as does the higher temperature reaction that
proceeds at red heat. With insufficient water present the reaction will actually accellerate from the wet mode reaction and transition to the
pyrolytic high temperature reaction as the temperature moderating water is boiled away by the heat of reaction.
More interesting yet, is that the ferrous sulfide product gotten from the wet method reaction is more highly reactive with HCl at lower temperatures
to more vigorously produce H2S as would be desirable for an H2S generator use. The byproduct ferrous chloride has typically been discarded, however
in this case the byproduct would itself be a useful reducing agent and should not be discarded. Likewise is the ferrous sulfide itself a useful
reducing agent if it were to be used directly, instead of being used as a reagent for H2S generation.
Much simplified types of apparatus can be practical for the occasional use where no continuous supply or on demand generator is needed by an
individual experimenter. Much of the literature seems to describe equipment designs intended to produce H2S in substantial quantiities for uses which
require larger quantities of H2S than an individual experimenter would need, but the materials and methods having advantages for the large scale are
adaptable also to the small scale with simpler implementation.
Uncle Paul, Emile, and Jules
http://www.mainlesson.com/display.php?author=fabre&book=...
Attachment: Hydrated Protosulphide of Iron from Hand_book_of_chemistry, Gmelin Vol. 5, pg 230.pdf (189kB)
This file has been downloaded 950 times
Attachment: Pages from A_Dictionary_of_Chemistry_and_the_Allied pg400.pdf (289kB)
This file has been downloaded 1086 times
Attachment: Pages from Journal_of_the_American_Chemical_Society.pdf (249kB)
This file has been downloaded 771 times
[Edited on 14-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Small Correction:
| Quote: |
This was what I wrote a couple of posts up. It should have said:
If the generator had not plugged, the 8g of sodium hydroxide would have been converted nearly completely to sodium hydrosulfide. Since Sodium
hydrosulfide is the product of the half neutralization of H2S and forms an equilibrium in aqueous solution with sodium sulfide and sodium hydroxide
the equilibrium can be pushed back in favor of sodium sulfide by adding more sodium hydroxide to the solution. Na2S + H2O <---> NaHS + NaOH
|
Found some more good stuff for hydrogen sulfide generation I see.
[Edited on 25-12-2014 by Bert]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
| Pages:
1
..
6
7
8
9
10 |