| Pages:
1
..
72
73
74
75
76
..
104 |
rka
Harmless

Posts: 2
Registered: 26-5-2017
Member Is Offline
Mood: No Mood
|
|
chemistry
How to, chemically and not electrolytically, get Ag from AgSCN powder?
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Heat.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Boiling AgCl in aqueous sugar is frequently performed (I have done it successfully a few years ago) to retrieve metallic Silver. This may provide a
path here, but be wary of products produced and disposal issues thereof.
Please note, not all sugars are equally effective in this reaction! See discussion at https://www.finishing.com/195/29.shtml .
|
|
|
gdflp
|
Threads Merged
27-5-2017 at 05:04 |
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Can anyone point me in the direction to find some literature about glutamate dehydrogenase inhibitors? I haven't been able to find anything so far.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
google scholar: glutamate dehydrogenase inhibitor / inhibition. Gives various hits. Or are you looking for something specific?
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
There was a thread/discussion on the board somewhere or other regarding seperation of sodium chlorate from sodium perchlorate using acetone. It was
mostly figured out by (I think) Hennig Brand.
Can someone link me to the thread. I simply CANNOT find the thing.
Thanks,
Yob
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Element mirrors
The silver mirror (Tollen's reagent) is very well known.
I am interested in attempting the same thing with different elements. I have heard that something similar can be done with copper using hydrazine as
a reducing agent.
I was wondering if anyone had any experience with mirroring other elements. Specifically bismuth.
J.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Question
Around what concentration does sulfuric acid cease to be an effective drying agent? More specifically, can my drain cleaner sulfuric acid (~93%) be
used to dry bromine?
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
Question.
Today was making a solution of copper (II) chloride via mixing a 1:1 ratio of HCl and 3% H2O2 and adding that to copper. I have done this before but I
had a little bit of a different result this time. I left it for around two hours and it soon became this dark brown liquid. I tested it by adding a
few drops to aluminum and it made the famous vigorous reaction between CuCl2 an Aluminum. I also dilluted a small sample and it turned into an olive
green color.
My guess on what it is is a saturated solution of copper (ii) chloride or that it's just really contaminated with copper (i) chloride. What
chemical do I have here???
|
|
|
cant think of a username
Harmless

Posts: 6
Registered: 14-4-2017
Member Is Offline
Mood: No Mood
|
|
question
hi,so i just rewatched nurdrage's video for making luminol and i was wondering what exctly happens when he mixes aluminium and sodium
metabisulfite?does it form sodium dithionite in situ? or is it something else.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by TheNerdyFarmer  | Today was making a solution of copper (II) chloride via mixing a 1:1 ratio of HCl and 3% H2O2 and adding that to copper. I have done this before but I
had a little bit of a different result this time. I left it for around two hours and it soon became this dark brown liquid. I tested it by adding a
few drops to aluminum and it made the famous vigorous reaction between CuCl2 an Aluminum. I also dilluted a small sample and it turned into an olive
green color.
My guess on what it is is a saturated solution of copper (ii) chloride or that it's just really contaminated with copper (i) chloride. What
chemical do I have here??? |
I think the dark brown is either the tetrachlorocuprate(II) ion, or a complex ion containing both copper(I) and copper(II).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
TheNerdyFarmer
Hazard to Others
  
Posts: 131
Registered: 30-9-2016
Member Is Offline
Mood: No Mood
|
|
Is there any way to know the percent of copper ii chloride so I can try and extract it?.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
If you dilute it sufficiently, any copper(I) chloride should precipitate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
RogueRose
International Hazard
    
Posts: 1592
Registered: 16-6-2014
Member Is Offline
|
|
Determinig KCl vs NaCl solution - easy way to differentiate?
Is there any way to test a solution to see if it is sodium or potassium chloride
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
A flame test is a good way to distinguish, if it is pure. A wooden splint dipped in KCl solution and held in a flame will give a light purple color,
while an NaCl solution will give a bright yellow-orange color. The presence of sodium will cover up the color from potassium though, so it isn't
perfect.
Another way that comes to mind is that some potassium salts are much less soluble than their sodium analogues, such as with chlorate and perchlorate.
So if you happened to have some sodium chlorate, you could add some of your solution to a reasonably concentrated solution of it and see if you get a
precipitate.
|
|
|
RogueRose
International Hazard
    
Posts: 1592
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by zts16  | A flame test is a good way to distinguish, if it is pure. A wooden splint dipped in KCl solution and held in a flame will give a light purple color,
while an NaCl solution will give a bright yellow-orange color. The presence of sodium will cover up the color from potassium though, so it isn't
perfect.
Another way that comes to mind is that some potassium salts are much less soluble than their sodium analogues, such as with chlorate and perchlorate.
So if you happened to have some sodium chlorate, you could add some of your solution to a reasonably concentrated solution of it and see if you get a
precipitate. |
Thank you, the flame test should be sufficient to verify what the solution is as it should be a pure solution (99%+ purity of original salt mixed with
distilled water). Thanks again!
|
|
|
RogueRose
International Hazard
    
Posts: 1592
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by zts16  | A flame test is a good way to distinguish, if it is pure. A wooden splint dipped in KCl solution and held in a flame will give a light purple color,
while an NaCl solution will give a bright yellow-orange color. The presence of sodium will cover up the color from potassium though, so it isn't
perfect.
Another way that comes to mind is that some potassium salts are much less soluble than their sodium analogues, such as with chlorate and perchlorate.
So if you happened to have some sodium chlorate, you could add some of your solution to a reasonably concentrated solution of it and see if you get a
precipitate. |
well I took a long wooden match stick, dipped it into the solution and put it into the blowtorch flame. I had 2 known (NaCl and KCl) solutions and an
unknown. the NaCl gave a nice yellow/orange flame almost as if I was adding an accellerant to the flame. If put at the base of the flame, the length
of the flame turned yellow/orange. When I did the KCl I still got a similar flame, but it didn't seem as "energetic" - if that makes sense, it
woulnd't propagate the flame the same way when placed at the base (all solutions are super-saturated at room temp).
The unknown does look more similar to the KCl but it is not a purple flame except maybe a couple mm immediately off the match stick.
The purple wasn't easily noticable in the known KCl solution as they all gave a yellow orange flame but the NaCl never produced the small purple near
the wood.
Is this the results I should expect? I was concerned that maybe the wood was a little different to give the purpleish color.
The sodium flame was impressive though!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The trouble with the flame test is that soooo many things are contaminated with just enough sodium to overpower the other colours. A common trick in
the old days (when people relied on the test) is to view the flame through didymium welder's glass, which blocks out the yellow quite selectively. Or
using a platinum wire instead of a wooden stick.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
first find something to hold your sample in the flame which does not itself give a yellow flame ... surprisingly difficult.
Nichrome resistance wire works ok, I think nickel spatulas but I'm not sure,
carbon often gives a similar yellow.
even then, if the 1% impurity is sodium (quite possible) then it may be enough to show as sodium.
I have tried borax bead tests, if I have nothing else to do I may re-visit .... useful but tedious.
https://en.wikipedia.org/wiki/Bead_test
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Even so, if his sample was sodium chloride, or contained a substantial amount of it, it would have been very obvious. I think it's safe to conclude
that the unknown is KCl, assuming that it could either be NaCl or KCl, and not anything else.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
true,
I just thought that the borax bead test was worth mentioning
(plus, I was showing off the entire extent of my knowledge in this area 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
@RogueRose
If you are really desperate, make a sodium cobaltinitrite solution. Potassium cobaltinitrite is insoluble in water and will precipitate out, while
sodium cobaltinitrite won't. But it may contaminate the potassium chloride solution, so perform this test in a separate reaction vessel.
[Edited on 6-11-2017 by ninhydric1]
|
|
|
The Aussie
Harmless

Posts: 1
Registered: 10-6-2017
Member Is Offline
Mood: No Mood
|
|
Question
For a distillation, is it possible to set up a Syphoning system if you don't have a water pump on hand, would this work? I was thinking something
like this and just lifting the water at the bottom to the top by hand.
thanks.
PS this is my first time here so yea, sorry if this has already been discussed, although i did look , I could not find anything.
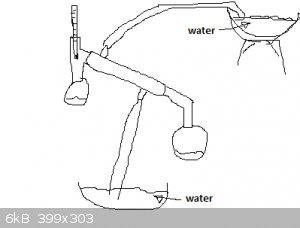
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I'd be more inclined to use a regular tap.
A siphon is not going to be a recirculating system unless you babysit it and tip the water back into the top bucket.
Also, the flow-rate will diminish as the had decreases.
It just all seems awkward. Either spend $20 bucks on a pond pump or plan on flushing some water down the drain.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
A syphon is certainly a way to cool a condensor. The flowrate can be easily adjusted by raising the upper basin. This allows you to control the
temperature of the output quite precisely. As has been said above you need to baby-sit this setup and be prepared to reestablish the syphon several
times per run. Aquarium pumps are the way to go.
|
|
|
| Pages:
1
..
72
73
74
75
76
..
104 |