| Pages:
1
..
5
6
7
8
9
..
48 |
Splinky
Harmless

Posts: 19
Registered: 9-2-2008
Location: Elsewhere
Member Is Offline
Mood: No Mood
|
|
Well, my current cell is a two liter soda bottle. The other jar didn't look to good on closer inspection. I think I'll try to get a bucket that
holds a few gallons so I can just have week long runs over the winter and stock up for seasons more hospitable to pyrotechnics.
The only problem I'm experiencing is the build up of junk on my electrodes and a thin layer on the stuff surrounding the cell, so I should probably
put a cover on my next one.
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
A possible alternative to your soda bottle might be a PET water cooler jug. The ones we have here are ~5 gallons and are PET of reasonable thickness.
|
|
|
Splinky
Harmless

Posts: 19
Registered: 9-2-2008
Location: Elsewhere
Member Is Offline
Mood: No Mood
|
|
Aren't those insulated at least partially? Even with temps in the thirties at night my cell has stayed pretty warm, would higher temperatures have
any negative effects or just help KCl stay dissolved?
I decided to go with the jar, it's about four liters and if it holds up well it should suit all my needs.
Are there any further precautions I should take if I make barium/strontium/calcium chlorate as well? I seem to remember reading somewhere that some
MMO anodes don't do well with barium compounds, but I don't remember where that was.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Warm temps (to a point) are desireable. What sort of current are you seeing? With 50 amps, I had to immerse my 6 liter cell in a vat of water, and
direct a fan across the surface of the water. Otherwise, the temperature would climb above 70 or 80 degrees. The evaporative cooling system took the
whole assembly below 50.
If you have enough current, I don't think you need to worry about warmth.
One other consideration in large systems... solutions heavy in salts tend to form aggressive temperature gradients. If the electrodes are near the
top, as most are, you will heat just the top layer, and the hot liquor will remain near the top, while the bottom remains cool. If you route some
stiff polyurethane tubing, or better yet PTFE tubing (don't use nylon) through the lid, to the bottom, and inject air using a strong aquarium airpump,
you will break up the layers and form a nice, homogenous liquor, keeping evolved chlorine gas in contact with the solution longer, which is
desireable. You'll have a healthier, more efficient system with mixing and agitation.
|
|
|
Splinky
Harmless

Posts: 19
Registered: 9-2-2008
Location: Elsewhere
Member Is Offline
Mood: No Mood
|
|
I'm only running it with a six amp battery charger. If I'm reading the meter on it right it says it's drawing upwards of eight amps, but it's still
nowhere near the 40+ amp cells that people are making. I think I'll look around for a supply more in the area of 20-30 amps if I can find one cheap.
Would it be efficient to just have a plate of titanium/stainless steel on the bottom of the cell to act as a cathode? That would provide some
agitation throughout the whole container.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Your electrode spacing is going to determine what current your system will create, among other parameters. For example, if your battery charger is
6V, and capable of 30 amps, but you are seeing 6 on the meter, by moving the electrodes closer together, you are reducing the resistance of the cell.
The result... the same 6V will now show 10 or 15 amps.
If you place your cathode on the bottom, you might end up with too much spacing, requiring additional voltage to achieve the same current. If you can
get away with it, though, it might not be a bad way to obtain some "free" circulation.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Splinky
Would it be efficient to just have a plate of titanium/stainless steel on the bottom of the cell to act as a cathode? That would provide some
agitation throughout the whole container. |
Hello,
Landing the cathode at the bottom of the tank will give you a uneven current density distribution on the anode.
Greater current density on the bottom of anode than the top.
If you must have a cell that is much taller than the anode/cathode assembly you could place the anode/cathode assemble into a plastic pipe that is
about a cm or so off the bottom of the cell (or have holes drilled at the bottom) and a cm or so under the liquid at the top. You will then get
circulation from bottom to top inside pipe (opposite outside) of cell (a toroid) via the H2 released.
According to www.corrpro.com MMO should not be used in electrolytes containing Barium or Cadmium.
According to Encyclopedia of Chemical Processing and Design, Vol 51, 1995 page146, Barium is a poison to Noble Metal or Noble Metal Oxide anodes and
may even reduce there lifetimes.
According to US Patent 7,250,144 (July 2007) Fluorine additive can damage Chlorate anodes (probably MMO, since it is a modern patent).
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Speaking of patents, I was googling "Industrial chlorate ph control" and found a new patent (Sep 08) claiming the use of phosphate as a buffer allows
the industry to do away with chromium in all its forms. The inventors did several experiments, and the best results were obtained with a specially
treated carbon steel cathode, electrodeposited with Mo and Fe. Phosphate ions were introduced, and apparently it did exceptionally well. A second
experiment used untrreated cathodes, and still did very well.
I can see phosphate doing something to those carbon steel cathodes; "parkerizing" comes to mind. But what interested me was the use of phosphate to
help buffer and keep pH under control.
"3g/l sodium acid phosphates (Na2HPO4 & NaH2PO4)" made up the buffer.
Any thoughts on this? Do you think it might be useful? I refuse to touch Chromium in any form, and NaF doesn't excite me either. How could one
prepare the sodium acid phosphates? And what might it do to subsequent processing of the product?
The patent number: US20080230381
http://www.freepatentsonline.com/20080230381.pdf
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I'm sorry, I meant to also post this: A doctoral thesis on the electrochemistry of the chlorate process. 119 pages of doctoral yumminess. First
glance indicates it will be an excellent resource for the dedicated amateur.
www.diva-portal.org/diva/getDocument?urn_nbn_se_kth_diva-344...
I wish I had written it. Enjoy!
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
A nice piece of bedtime reading.
Would not a sup of Phosphoric acid (rust proofer) be OK.
Persulphates have also been used with Lead Dioxide to stop cathode reduction of products.
I don't know if persulphate buffers the solution.
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I was thinking that the easiest way to create the buffer would be to mix phosphoric acid and NaOH and adjust the ratios to achieve a pH of around 6.0,
but it would be more economical to have the mono and disodium phosphate salts at hand, determine a correct ratio, and add the salts during
preparation. Where one would obtain these salts at an economical price, I have no idea. Anyway, it'd definitely be for a future experiment.
I love the way these patents say things like "current efficiency dropped to 90%; unacceptable." I'd kill to get 90% efficiency! 
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Chemsavers does have the disodium phosphate at $31/500g.
Reading that hot thesis.. I wonder if that chick is hot?
I see a potential problem for pH control - "The pH of the electrolyte varies from about 4 at the anode to 6-7 in the bulk of the solution and up to
high values - in the range of 13-14 - at the hydrogen evolving cathode."
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
@Tentacles
I don't understand what you mean by the above being a problem.
When we are controlling the pH, it is the pH of the bulk solution we are interested in. This is where 'chemical chlorate formation' will take place.
(We get no 'chemical chlorate formation' in the non pH controlled cell, only 'Chlorate made by electricity'.)
This is why, in pH controlled cell, it is best to have either a fairly large Anode/Cathode chamber (plenty of room away from the anode) for the 'bulk
reactions' (chemical Chlorate formation) to take place or to have a second chamber for the 'bulk reactions' to take place.
You will always have a low pH at the anode surface and a high pH at the cathode surface. We can control the bulk solution area.
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I would also assume that those varying pH values are in gradients... the closer to the electrode, the higher/lower the pH, and as the electrolyte
moves, from either mechanical stirring or heat, it'll all mix together rapidly to an averge pH.
I have done a lot of thinking on pH the last few days. I know the industrial units have a pH electrode immersed constantly. For an electrode to
survive any length of time, and not be fatally poisoned by the liquor, it's going to need some excellent (and expensive) engineering, and even then, I
think the probable lifetime of a pH electrode can be measured in a batch or two.
Since those guys are making multi-ton batches, they can afford a $300 pH electrode every so often. Their electrode cost per kg is quite low.
Whereas OUR cost, with the same $300 electrode, is too high to contemplate.
I'm guessing a single-junction aquarium electrode ($15) will be fatally poisoned in just a few hours. What's the answer? I have no idea. There's no
point in having a controller if you have to manually immerse the electrode every hour or two, cycle in some acid, then remove the electrode to prevent
contamination. An advanced setup might have some sort of PTFE solenoid, which would open at intervals, flood a pH electrode chamber, acid cycles on,
then the chamber is drained... and now you have an electrode which is high and dry, also not a good thing.
I may be completely wrong... maybe a modest double-junction pH electrode will last for years with minor care. I hope so, but I've got a feeling they
will die quickly as an accurate measuring device.
Maybe one way would be to monitor a cycle with a cheap electrode, and note what sort of HCL dosing schedule (so many ml/min) would keep things
reasonable. Once that is known, you can probably be successful in simply replicating that dosing schedule without any sort of pH monitoring.
I've got the controller; the dosing pump is coming, and an inexpensive electrode is as well. A pH electrode is the ONE thing I am very leery of
buying off eBay. They're either used and suspect, or NIB old stock, also suspect, since these things have a shelf life. That is why I am interested
in nontoxic buffering like the phosphate salts, to ease the process a little bit.
Sorry for the rambling post. I'd like to get my rig running in a day or two, but I'm not ready to control pH yet.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
You might be pleasently surprised by how well the dose + measure strategy may work. It worked OK for me in a very simple cell. The pH started to go
astray as the cell became depleated of Chloride but that is not going to happen with your system which will be very stable and predictable when you
get it up and running for a few days.
Two measurements per day (I guess) would be easily enough to keep an eye on pH after stability has been achieved.
They sing the praises of Reflex probes here:
http://www.southforkinst.com/ph_electrode_facts.htm
Some times in industry the pH is controlled by bubbling in Chlorine.
The pH can go no lower than approx. 4 if a dosing mistake is made.
Not suitable for the garage guru.
I have heard of a guy (long time ago) suggesting (or actually doing, not too sure) that pH could be controlled by bubbling in CO2 to the cell. Your
not going to have a CO2 source around though, for to do that. He reckoned that the continous bubbling of CO2 would control pH at around neutral
without needing a pH probe. Would there be enough CO2 in air?
Also regarding recharging with KCl. Would KCl dissolve in HCl acid ?.
It will depend on how much acid you have to add but if you were to add KCl to the acid (if it will dissolve), then you could kill two birds with the
one stone.
Dann2
|
|
|
Splinky
Harmless

Posts: 19
Registered: 9-2-2008
Location: Elsewhere
Member Is Offline
Mood: No Mood
|
|
I don't think the KCl dissolved into hydrochloric acid would work too well as hydrochloric acid is just HCl dissolved in water. Unless the HCl
significantly improves the ability of KCl to dissolve in water it would just be like adding a bit of saturated KCl solution.
I think a CO2 welding/beverage tank would hold enough to slowly bubble gas into a cell for several days, if you're willing to buy a tank (in the area
of sixty bucks) you could probably manage a few runs in a large cell per charge. Refills are between ten and twenty dollars.
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
I read through the first section of that paper today, except the bits I don't understand (about half the charts, anyways). Interestingly, it mentions
commercial DSA chlorate anodes as being "worn in" after THREE YEARS of use (continual, or nearly so, I would expect). It also has some interesting
info on the coatings, a RuO2/TiO2 blend. Some of them apparently have a RuSn3O4 or something dopant added. Thicker layers prove to wear out faster
than a somewhat thinner layer, etc. I'm sure everyone here has read through considerable portions of it. The references will be very useful to look
through, I think.
Swede, her diagram of a chlorate cell is very close to what you're building. Her test cell was 100ml cell (chamber?) volume (no idea of solution
volume) and she used a flow rate of 2.2L/min through the cell chamber. That's serious flow, but some of the tests she ran were at a staggering
3.8A/cm^2! (38KA/m^2).
dann: I just sent those refex guys an email. Who knows, maybe they're suckers for people who want to make strong oxidizers?
I'm not sure I see the point of adding any acid besides HCl to adjust the pH of solution - all you're going to achieve with CO2 is to make (salt)
carbonates, when what we want is chlorides. The pH goes up because the chlorine is evolving away (as we all know). Besides, you really aren't going to
get much carbonic acid to dissolve/form in a hot electrolyte, without pressure. Carbonated chlorate/hypochlorite solution, anyone? Don't bump the
tank!
[Edited on 2-11-2008 by tentacles]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Swede
I have done a lot of thinking on pH the last few days. I know the industrial units have a pH electrode immersed constantly. For an electrode to
survive any length of time, and not be fatally poisoned by the liquor, it's going to need some excellent (and expensive) engineering, and even then, I
think the probable lifetime of a pH electrode can be measured in a batch or two. |
Forgive me for asking what
may be an obvious question, but do you need to measure actual pH, or will some proxy for pH work? The core of a regular pH electrodes is a proton
membrane, which passes only H<sup>+</sup>, and so measures pH directly. But for a known process, where all the species in solution are
known, it seems that total conductivity, perhaps without a special membrane, would correlate adequately to the process parameter involved.
Idea Zero: Use ordinary conductivity. If this is adequate, why do anything more complicated?
Idea One: Use a hardier membrane with less selectivity. If you were to use a membrane that passed mono-atomic species but not polyatomic ones, this
would seem to give at least some idea of the free chlorine in solution. Surely that's worth something. I can't say now whether it's sufficient.
Idea Two: Use transient current behavior. Ordinary pH electrodes use DC currents, which could be done easily in days past. What was not so easy back
then was to get detailed measurement of the transient AC response; that's pretty easy to do nowadays with fast A/D converters and microprocessors. The
physical principle is that the depletion region around an electrode acts like a capacitor. The rate of discharge is related to the diffusion constants
(read, masses) of the species adsorbed electrically onto the surface of the electrode. So you'd first have a charging cycle that used a potentiostat
to bring the electrode to a known state. The next cycle would be to discharge the electrode, <i>i.e.</i> suddenly change the voltage
(probably by reference to an internal sample-and-hold, rather than solution). Observing the rate of discharge gives an idea about what species are
present. A third stage cleans the electrode with AC before going back to the beginning. Different charge and discharge potentials should give a pretty
good sample of the various species present. While the software to run such a device would be pretty extensive to get right, the underlying
electronics, while not really simple, are still pretty modest.
Idea Three: Combine with density measurement. Apropos another recent thread, an ultrasonic range finder can be used to measure density. In combination
with other conductivity measurements that are not in themselves definitive, density may tip the scales.
Idea Four: Measure electrode capacitance by resonance. Operate an electrode at some DC bias current with a combination potentiostat/galvanostat.
Impose upon that bias current an AC signal (likely in the RF range, given the total charge on the surface of a small reference electrode) that uses
the electrode capacitance as part of the oscillator. This yields a frequency dependent upon the local electrode conditions. Sweeping over the DC bias
by controlling the potentiostat allows sampling over different regimes of ion adsorption.
Idea Five: Use a differential reference electrode which is a tiny loop of platinum wire connected to twisted-pair leads.
I'm making no claim that any of this is any better than a commercial pH probe. I'll consider this posting successful if it jogs a good idea.
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
@ Swede
Thanks for that excellent doctoral thesis. It's years since I made any chlorate but that work dispelled a few myths I had accumulated about it. Such
as thinking that the Cl- concentration should be as high as possible. The ideas presented probably have meaning for other MMO anodes such as those
discussed in this forum. I wish it had a bit more about graphite anodes, though.
The amateur does not have the same concern with power efficiency that commercial makers have, but current efficiency is important to us, because of
the time taken for electrolysis. The other factor is electrode erosion. I'd love to get 1kg/ton erosion on graphite! in my efforts it seemed like
50kg/ton!
Regards, Der Alte
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Just in case you folks run out of reading material, I attach another Thesis.
This one was pluged on the board some time ago so you may have seen it before.
Going back up this thread a bit we were talking about having no Chromate in the cell.
On page 4 of the thesis it states:
____________________________
Reduction of chlorate is highly dependent on cathode material and takes place on iron whereas
cathodes of Co, Ni, Mo, Ti, Hg and C do not show any activity for chlorate reduction [11,12].
_____________________________
So that just leaves Hypochlorite being reduced (destroyed) I guess.
On page 36 it states:
______________________
Hypochlorite reduction is limited by mass transport at higher current
densities, and the increase in limiting current density seen in Figure 14 may be a mixed limiting
current due to limited supply of H+ and transport-limited hypochlorite.
_________________________
What that is saying (I think and hope!) is that if you have a high current density on the cathode (a cathode with a small effective surface area) then
Hypochlorite reduction at the cathode will be minimized.
Yittrium is also stated to have a similar effect to Chromate and is less toxic but it would be better to not have any of these additives in the cell
(and product) to start with.
About monitoring the pH.
Perhaps another possible way would be to monitor the amount of Chlorine gas that is coming off the cell. I think this would be a good indication of pH
value.
Are there any cheap gas monitors that can be purchased?
I have read somewhere that industrial cells have been operated at a slightly higher pH than would be the optimum for max. CE, in order to have less
Cl gas to deal with in the exhaust gases from the cells. Going more green, as it were, or literally less green (pun intended).
As pH goes up less Cl comes out.
Don't know if that qualifies as a good idea (joged) or a brain seizure but if it was easy to implement it should work.
Dann2
Attachment: DSA Thesis.pdf (2MB)
This file has been downloaded 1106 times
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I like the direction this discussion is taking... altenate methods to do what industry takes for granted. I found yet another patent (I hate reading
patents because it's often hard to find good nuggets in them, but they are there) that makes use of a tungsten electrode set and appropriate
electronics to measure chlorate concentration, much like watson.fawkes mentions. Further, I am confident that there are ways to determine chloride
concentration (end of run) conditions based upon voltage/current plots, even when efficiency may not be known. Logic says that as the species change
in the system, the voltage required to create a given current (with a good supply capable of constant current operation) will follow a predictable
path. What I've seen with my own system, starting with potassium salts, is a concave voltage curve... it starts high, drops, then begins to rise once
more as the chloride is depleted. As WF says, conductivity may be a useful measurement that is overlooked.
My point is, with enough research and some hands-on experimentation, things like quantitative analysis, pH control, and other pressing issues, can and
will be overcome.
Dann2, I laughed when you pointed me towards the Reflex electrode. I have literally been researching poisoning-resistant pH electrodes for weeks
without finding anything like that. I've come up with "Triple-junction, glass-domed, PTFE membraned, long life electrodes" stuff like that, but
nothing that flat-out says "It cannot be poisoned." I am going to email or call them as well and see what they have. I will not hesitate to buy an
expensive pH electrode if I know that it will survive. Worth every penny, IMO. Thank you for the resource.
But for setups without a controller, I like the concept of simply adding calculated quantities of HCl, and doing periodic checks with a pH meter. I
did not realize that there was a calculable quantity of HCl consumed given a known current. Since we know our current (ammeters are cheap) then we
should know how much HCl to trickle in. A dosing pump set up on a timer could administer X ml per hour, and all you have to do is check on it once in
a while.
I am interested in efficiency... it tells me that I'm doing it correctly. The runs where I calculated my efficiency, it was at best 61%, maybe a tad
bit higher. That was with no pH control at all beyond inadequate shots of HCl using a wash bottle. I think a well-run home chlorate cell can do 75%
to 80%, and if I can get there I'd be delighted. The other good thing, if you know your efficiency, and it is reasonably constant batch to batch, you
can use math rather than tedious chloride quantitative analysis to determine end-of-run.
I am going to finish plumbing today... I need to get more HCl from the pool store, and rig up some sort of drip system, before I fire it up. I don't
want to proceed without at least a crude way to keep the pH in check.
Again, sorry for yet another "stream of consciousness" post, but I am delighted to find a bunch of smart guys here with a common interest. Wish me
luck!
Oh yes, I finally figured out a good way to create a swappable anode "cartridge". This was baffling me for some time, but the end result is simple.
I created a round slug of CPVC, slotted it 98% of the way through for the anode strap. I drilled 3 or 4 holes through the strap where the CPVC circle
would fit, and then glued it in. The holes are critical... PVC cement does not stick at all to Ti. The holes form a PVC "bridge" between the
hemispheres, and keep everything secure.
Next, the anode strap + CPVC slug was glued into a rimmed carrier that has a viton o-ring, not installed in the pics. This carrier is installed in
the cell lid from below, and an aluminum block is clamped on the topside to fix the strap in place... it cannot move vertically. This will replace
the "sculpey" PVC clay plug that I had tried earlier.


|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
One more figure for acid consumption in Sodium Chlorate process:
http://books.google.ie/books?id=_R00NqWST6MC&pg=PA233&am...
Encyclopedia of Chemical Processing and Design
Vol 51 page 184
50kg HCl per ton Sodium Chlorate.
It does not say if they mean 35% HCl acid (or perhaps 100% equivalent).
Thats 50 grams per KG Na Chlorate
What is the No. of the patent showing the set up for measuring Chlorate conc.?
I like that chunk of Al. It will be a great heatsink. If you really have a stubborn heat problem at this point you could cut slots in the block, just
like a regular heat sink.
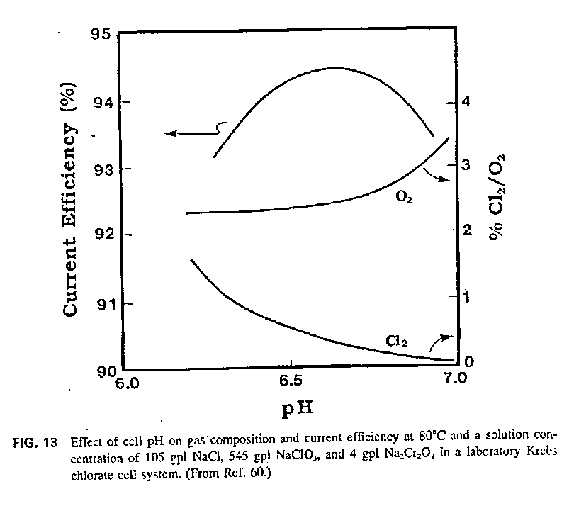
In regards to monitoring Cl2 coming from cell see pic. below. It is from E. of Chem Proc. and Design.
Perhaps in conjunction with this type of device it may do instead of pH monitoring.
http://cgi.ebay.co.uk/Industrial-Scientific-CL266-Chlorine-G...
Cl coming from cell may/will also be a function of things other that pH so you may have to keep cell parameters constant (eg. temp) when using this
method.
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
dann2, the patent (uElectrodes for chlorate concentration)
http://www.freepatentsonline.com/5948236.pdf
The language was beyond my abilities, but I did only glance at it, not study it in detail. It sounds like it will require some advanced equipment and
signal processing.
I'm guessing that 50g/kg of HCl is the actual mass of HCl, so we need to take normal dilutions into consideration.
HCl density: Here's an interesting table:
http://www.starch.dk/isi/tables/hcl.htm
It states at 38% by weight acid (normal pool acid) you get 451.6 g/l, or 0.4516 g/ml, of HCl.
At 100% efficiency, it requires 161 ampere-hours to convert 1 mole of chloride to chlorate. For potassium salts, that converts to 1313 ampere-hours
(AH) per kilo; sodium salts, 1511 AH/kg.
Let's determine the acid per AH needed at 100% efficiency. The MW of sodium chlorate = 106.5; there are 9.39 moles/kg. 50g acid is needed for each
kg sodium chlorate. 50g HCl / kg sodium chlorate converts to 5.33g HCl per mole.
161 amp-hours creates 1 mole of chlorate. Thus, you need 5.33g acid per 161 AH, or 0.0331 g HCl per AH, undiluted.
With 38% pool acid at 0.4516 g/ml, you need 0.0331 / 0.4516 = 0.0733 ml/AH acid.
I hope my math is correct here. If your cell runs at 50 amps, you should set up a drip or dosing system that delivers 50 X 0.0733 = 3.665 ml per hour
of 38% HCl. Not as much acid as I would have thought, frankly. If you use more diluted acids, the table will tell you how much acid per liter is
there.
Summary:
100% acid per amp-hour: 0.0331 g
38% acid per amp-hour: 0.0733 ml
Anyone see anything wrong with my analysis? It assumes 100% efficiency, but since the acid goes in along with the electricity, if you have to run
your cell longer due to normal inefficiencies, you'll be applying extra (needed) acid.
Dann2, thanks for bringing this up. I think a rule of thumb like this (3.7ml/hour per 50 amps) will be very helpful, if it in fact works out that
way.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
I agree with your calculations.
With '100%' acid you need 39.7ml acid per day.
If you used 12% acid you would need 331 ml per day. (hope I got that right).
A figure that I give (from my jamjar type cell that was pH controlled) is not too far (plus 25%  ) from this figure. I calculated on post of 29-0-08 that you would need 420 ml 12% acid (from the jamjar data) for your
cell running 50 amps. ) from this figure. I calculated on post of 29-0-08 that you would need 420 ml 12% acid (from the jamjar data) for your
cell running 50 amps.
If you have room in the cell for the extra water you would be better off using a lower % that 38%, as each time you drop a drop of the 38% stuff into
the cell you will get an immediate reaction at the liquid surface and a smell of Cl2.
You could inject into bottom of cell and that would help. Perhaps it is a very minor issue.
Dann2
[Edited on 3-11-2008 by dann2]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
I finally received the MMO anodes Swede kindly made up for me, and I spent the morning clearing off my work table and getting out the LD plating
apparati. I can't seem to find my stir bar, though, I can probably borrow one this weekend but in the mean time I think I will fabricate one with some
HDPE tubing and rod magnets.
edit: Got a reply back from the Refex guys. It's not pretty:
Hello Brian
Refex probes can be used in chlorate and perchlorate manufacturing
processes. Their advantage is a unique non-porous reference junction that
prevents poisoning of the probe and extends life to up to 5 times that of
conventional porous junction and direct contact probes.
Refex probes are available direct from South Fork Instruments. Typical
price for a combination probe is approx $950.
Best regards
If anyone wants his contact info, just let me know and I'll U2U it. I assume by combination probe he means pH + ATC
[Edited on 3-11-2008 by tentacles]
|
|
|
| Pages:
1
..
5
6
7
8
9
..
48 |