| Pages:
1
..
5
6
7
8
9
..
23 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Anders Hoveland  | Yes, (NO2)3PhO(-) (+)NH3NH3(+) ClO4(-)
Where there are N2H6 (+2) cations and a mix of picrate and perchlorate.
Picric acid, as I have recently described in another post, likely will be able to react through a tautomeric ketone to condense with NH2OH... or with
NH2NH3(+) ! There is a diagram somwhere in "Quinone Explosives" topic.
Thus, may get the salt (NO2)3PhNNH3(+) ClO4(-)
This would be even more powerful, since it is the dehydrated form of the double salt I described.
Ph = phenyl obviously
|
True that phenol and trinitrophenol are able to transpose a -OH group for a -NH2 or -NH-NH2 group...
You would thus end up with trinitrophenylhydrazine...this would display rather acidic H on the first nitrogen close to the aromatic ring...the second
nitrogen will be less acidic and thus more basic allowing a "neutralization" by HOClO3 to provide (trinitrophenylhydrazinium picrate)
((NO2)3C6H2-NHNH3(+)ClO4(-)).
           
Thinking one step further is to allow trichlorotrinitrobenzene to react with NH2-NH2 to get 1,3,5-trihydrazino-2,4,6-trinitrobenzene...the later must
be a good candidate for making salts of HClO4, HIO4, HC(NO2)3, HC(NO2)CN and HN(NO2)2
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Do you really think dinitropyridine (a really deactivated aromatic ring) will react as you suggested with AlCl3 and Cl2?
You should check pyridine (heteroaromatic) chemistry before proposing such a pathway.
Do you really think NH2-NO2 will react so easily with chlorodinitropyridine? Isn't it too acidic for that? Usually such reactions occurs with basic
amines.
[Edited on 12-11-2011 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I wonder what is the structure of azidotetrazolate 2-oxide, by chance do you have an image of it?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Have a look at the spectra and structures yourself.
Attachment: 13068.pdf (758kB)
This file has been downloaded 1630 times
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Thank you Formatik, nice finding!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
What do you think about cellulose perchlorate? I wasn't able to find it mentioned anywhere, but I think it would be too unstable.

|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Cellulose perchlorate is indeed unstable and is subject to self-combustion-initiation.
When adding paper or cellulose to70% HClO4 you get a transparent gellous like stuff that detonates readily from flame. But being of the perchloric
ester familly, it is not something to store or to play with in significant quantities (> 2g).
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
What about sulfur diazide? SCl2 + 2 NaN3 ----> S(N3)2
Or some explosives derived from urea:
CON2H4 + Cl2 ----> CON2H3Cl + HCl
CON2H3Cl + NaN3 ----> H2N-C(=O)-NH-N3 - Quite interesting I think..
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Yesterday I got an idea of making biuret dioxime. I don't think it's impossible, but I can't find any info. Many interesting EM can be derived from
it, IMO.
Rest In Pieces!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
found this,
| Quote: |
Sulfuryl azide, SO2(N3)2 ... was made in 1922 by Curtius and Schmidt by shaking a suspension of sodium azide in sulfuryl chloride for 24 hr.
|
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I want to comment on this idea from Adas. The chlorine will not as readily substitute off because it is it the meta- position with respect to both
nitro groups. Probably better to use bromine instead. The resulting compound, if it forms, is also likely to hydrolyse with water and be significantly
more sensitive than other typical nitramines because the two nitropyridine functional groups would be much more electron withdrawing than the
methylene groups found in RDX. It may also be possible that the dinitrochloropyridine may be able to act as an oxidizer towards the NH2NO2, rather
than condensing with it. For example, both trinitrophenol and dinitroimidizole will oxidize hydroxylamine, with one of the nitro groups on the ring
being reduced to an amine in both instances.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
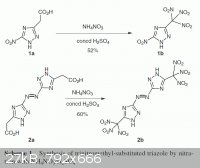
Someone might find this article interesting:
| Quote: | Synthesis of Trinitromethyl- and Dinitromethyl-Substituted Azoles Using Nitrate Salts in Sulfuric Acid
Venugopal Thottempudi, Jean'ne M. Shreeve
Synthesis 2012 (in press)
DOI: 10.1055/s-0031-1289736
Abstract
Synthesis of trinitromethyl and dinitromethyl-substituted azoles employing a mild and efficient nitration method has been developed using a mixture of
sulfuric acid and an inorganic nitrate salt XNO3, where X = NH4+, Na+, K+. This methodology exhibits clear advantages over the more popular mixed
acids nitration approach. Various nitro functionalities such as C-trinitromethyl, N-trinitromethyl, and dinitromethyl ester containing azoles were
prepared in moderate to excellent yields with reduced reaction times using this method. |
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Synthesis of Trinitromethyl- and Dinitromethyl-Substituted Azoles Using Nitrate Salts in Sulfuric Acid
article attached thanks to ayush
Attachment: Synthesis of Trinitromethyl- and Dinitromethyl-Substituted Azoles Using Nitrate Salts in Sulfuric Aciid.pdf (107kB)
This file has been downloaded 2046 times
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
A big THANK to Nicodem and Rosco Bodine  for this little golden nugget! for this little golden nugget!
 
I especially like the comparison and effect of extra groups or heteroatoms (nitrogen) onto the yield...
Introducing electrowithdrawing groups increases the yield...
Special attention can be set on the Ethyl 5-Tetrazolyldinitroacetate over 95% yield in less than 2 hours!
This opens me a lot of new ways of investigation....
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Trinitromethyl groups can add good oxygen balance, but the problem is significant increase in sensitivity, and thermal stability problems. My opinion
is that the trinitromethyl in its anion form (nitroformate) has much better stability, and thus would be much more practical.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
That is a gem of a paper.
The essential reaction scheme irrespective of the *azole , is the oxidation of the
acetic acid functional group into trinitroformate in a rather fascile manner. It begs
the question if this will work generally on any R-CH2COOH. A likely candidate to
investigate would be aromatic derivatives such as ,1,3,5- benzene triacetic acid.
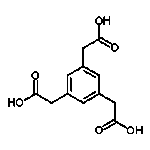
www.chemspider.com/Chemical-Structure.309530.html
www.guidechem.com/products/4435-67-0-p1.html
http://datasheets.scbt.com/sc-252412.pdf
a bit expensive at ~ $130 per 500 gm.
http://www.scientificlabs.co.uk/product/17383-500MG
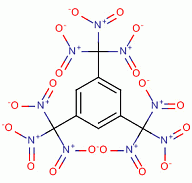
The intended result of this would be
1,3,5-Tris (trinitromethyl)- Benzene , CAS 210053-27-3
oxygen balance is , - 4.6
2 C6H3{C(NO2)3}3 => 15 CO2 + 3 CO + 3 H20 + 9 N2
Apart from Arylacetic acids other candidates are Alkyl Dicarboxylic acids.
http://en.wikipedia.org/wiki/Dicarboxylic_acid
By this process Succinic Acid would yield Hexanitroethane.
Succinic Acid (Butanedioic Acid) HOOC.(CH2)2.COOH
http://en.wikipedia.org/wiki/Succinic_Acid
Glutaric Acid (Pentanedioic Acid) HOOC.(CH2)3.COOH
http://en.wikipedia.org/wiki/Glutaric_acid
Adipic Acid (Hexanedioic Acid) HOOC.(CH2)4.COOH
http://en.wikipedia.org/wiki/Adipic_acid
Pimelic Acid (Heptanedioic Acid) HOOC.(CH2)5.COOH
http://en.wikipedia.org/wiki/Pimelic_acid
Pimelic can be synthesized from salicylic acid
http://www.orgsyn.org/orgsyn/pdfs/CV2P0531.pdf
Carboxylic acids can be made by oxidizing an alcohol group.
A typical reaction is:
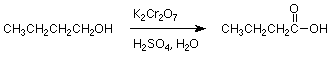
Syntheses of Tetracarboxylic Acids
Boris I Zapadinskii et al 1973 Russ. Chem. Rev. 42 939
http://iopscience.iop.org/0036-021X/42/11/R04
Ethylene Diamine Tetraacetic acid (EDTA) is available over the counter
from your health food store around $ 15 for 125 grams in capsules.
http://en.wikipedia.org/wiki/EDTA
.
[Edited on 3-4-2012 by franklyn]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by franklyn  | That is a gem of a paper.
The essential reaction scheme irrespective of the *azole , is the oxidation of the
acetic acid functional group into trinitroformate in a rather fascile manner. It begs
the question if this will work generally on any R-CH2COOH. A likely candidate to
investigate would be aromatic derivatives such as ,1,3,5- benzene triacetic acid. |
The electrophilic nitration of fairly active methylene groups is quite general, but the alpha-position of carboxylic acids is not generally considered
as particularly "active". That kind of triazole rings activate the methylene group more than a phenyl group does, so the nitration of phenylacetic
acids would not necessarily lead to the nitration of the benzylic position, at least not without first achieving the ortho and/or para ring nitration
which activates the benzylic position (an exception would be the radical nitration which should proceed readily on phenylacetic acid to give the
corresponding oxidation products).
The alpha-position of carboxylic acids can be activated also from the other (carbonyl) side - carboxylic acid anhydrides and halides enolize much more
easily and are thus way more activated than the corresponding acids. This is how acetic anhydride can be nitrated under relatively mild conditions: http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3...
The nitration of active methylene groups can be achieved with HNO3 like in the discussed article or the Org. Synth. example above. It can also be
achieved by the nitration of the preformed enolate by using certain nitrating reagents such as Me2C(CN)ONO2, alkyl nitrates, tetranitromethane, or
FC(NO2)3 like in the example of alpha,2,4,6-tetranitrotoluene synthesis described in the 1.8.1.3 chapter of Organic Chemistry of Explosives
(Agrawal & Hodgson, 2007). See its chapter 1.8 for a review of the nitration of active C-H groups.
PS: Those interested in the nitromethyl substituted triazoles might also want to check another article (DOI: 10.1007/s11172-009-0285-y) where another
ingenious methylene group activation strategy is used.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | The electrophilic nitration of fairly active methylene groups is quite general, but the alpha-position of carboxylic acids is not generally considered
as particularly "active". That kind of triazole rings activate the methylene group more than a phenyl group does, |
Although a methyl group on a tetrazole ring appears to be inert towards nitration, the addition of a carboxyl group [to the now methylene group] seems
to activate it to nitration.
Quote: Originally posted by AndersHoveland  |
Nitration of 5-methyltetrazole
various starting materials such as 2-methylimidazole,
2-methoxy-2-methyl-imidazolidine-4,5-dione,and2-methylpyrimidine-4,6-dione(4,5-dihydroxy-2-methylpyrimidine) were nitrated and then hydrolyzed to give
FOX-7 by somewhat different process. Since the methyl group was converted to dinitromethylidene moiety in all methods, nitration of 5-methyltetrazole
was attempted to afford 5-dinitromethylidene-1,4-dihydrotetrazole. But this reaction failed to proceed, and most of the starting material was
recovered.
| Quote: |
With the necessary intermediates in hand, we sought to construct the N2FOX-7 using decarboxylation and elimination. Thus, various conditions were
tried. When ethyl 5-tetrazolyldinitro-acetate was treated with water, 5-dinitromethyltetrazole was readily given. Hydrolysis followed by
decarboxylation took place completely within 2 h at 50 °C.
Synthesis and Characterization of High EnergeticTetrazole and Furoxan Derivatives, (Korea)
|
ethyltetrazolylacetate
N4HC-CH2-C(=O)-O-CH2CH3
The reason that the nitration of 5-methyltetrazole failed to proceed is probably the same reason that nitration of plain 1,2,3-triazole is essentially
impossible.* I suspect that the electron-withdrawing tetrazole ring pulls away electric charge from the methyl group, giving it a partial positive
charge and effectively shielding it from interaction with nitronium ions, which is the mechanism of reaction in most nitrations.
*In contrast, 4-nitro-1,2,3-triazole is easily both nitrated, and oxidized interestingly, by normal mixed HNO3/H2SO4 acids to
4,5-dinitro-1,2,3-triazole-N-oxide. The addition of one nitro group appears to activate the other CH to nitration.
|
[Edited on 3-4-2012 by AndersHoveland]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ Nicodem
" That kind of triazole rings activate the methylene group more than a phenyl group does "
As it does with triazine www.sciencemadness.org/talk/viewthread.php?tid=11195&pag...
Thanks for outlining the complexities involved. There is always more to this than
first appears to my unpracticed notions.
This is that other article cited by you.
www.springerlink.com/content/b451460133120472
.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Possible route to sulfuric diperoxide (S2O8)?
2 SO<sub>2</sub>Cl<sub>2</sub> + 2 BaO<sub>2</sub> ---->
O<sub>2</sub>S-(O-O)<sub>2</sub>-SO<sub>2</sub> + BaCl<sub>2</sub>
Rest In Pieces!
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
And what about organodinitramides?

But it's a shame that dinitramides are so difficult to prepare...
Rest In Pieces!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Chavez has his patent
Synthesis of Energetic Nitrate Ester US 20120108838
Attachment: US20120108838.pdf (248kB)
This file has been downloaded 976 times
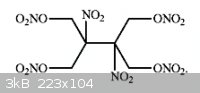
Recap from an earlier post in this thread
Synthesis of an Energetic Nitrate Ester
http://www.sciencemadness.org/talk/files.php?pid=141343&...
Thanks to 4 9 7 for this
.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
This brings us back to Axt post in 2004 about synthesis of Nitroisobutylglycerol Trinitrate from nitromethane and formaldehyde...
CH3-NO2 + 3 CH2=O --> (HOCH2)3C-NO2
(HOCH2)3C-NO2 + 3HNO3 --> (O2NOCH2)3C-NO2 + 3H2O
A very powerfull OB balanced HE...
I already talked about 1,2-dinitroethane as a possible source of the tetranitrate ester molecule you depicted...
2,3-dinitro-2,3-dimethylol-butan-1,4-diol tetranitrate.
O2N-CH2-CH2-NO2 + 4 CH2=O --> (HOCH2)2C(NO2)-C(NO2)-(CH2OH)2
(HOCH2)2C(NO2)-C(NO2)-(CH2OH)2 + 4HNO3 --> (O2NOCH2)2C(NO2)-C(NO2)-(CH2ONO2)2 + 4H2O
This ester has also a perfect OB like all molecules of the permethylolpolynitroalcane familly derived from polynitroalcanes H-(CH-NO2)x-H (x from 1 to
infinite).
As expected the later tetranitrate ester has a higer density, a higher VOD and less or equally sensitive than the former trinitrate ester... this goes
the same way as the predictive theory I work on for years now...
I can predict for the following members of that specific familly of perfect OB nitro-nitrate HE:
-that 1,2,3-trinitropropane will provide a pentanitrate ester that will be even denser and powerful than the previous member of the familly (the
tetranitrate ester here above)
-that 1,2,3,4-tetranitrobutane will provide a hexanitrate ester that will be even denser and powerful than the pentanitrate member
-etc
So if HMX has already the same VOD as the tetranitrate ester (9,1 km/s) ... can you imagine what would be the power and VOD of the pentanitrate and
hexanitrate?I guess > 9,5 km/s
This familly has the low impact and shock sensibility of PETN because each nitrate ester group is not directly vicinal and at least espaced by one
carbon atom free of nitrate group...
This effect is seen in 1,3-propanediol dinitrate (where position 2 is free of nitrate), in 1,3-butanediol dinitrate (in which position 2 and 4 are
free of nitrate), in 1,4-butandiol dinitrate (where position 2 and 3 are free of NO3), in polyvinylnitrate (where one on each two carbons is exempt of
NO3) and in PETN (where the central C atom allows separation of all 4 nitrate groups).
The effect is absent in 1,2 propandiol dinitrate, in 1,2 or 2,3 butandiol dinitrate!
[Edited on 20-10-2012 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Adas  | And what about organodinitramides?

But it's a shame that dinitramides are so difficult to prepare... |
It is a shame that chlorine atoms on aromatic rings doesn't substitute so easily... the way you wrote it...
Maybe if you add some withdrawing group on the ring...
2,4,6-trichloro-1,3,5-trinitrobenzene would be a much better option ... but the resulting (mono, bis or tris) dinitramide would be very unstable ... a
bit for the same reason trinitramide would be...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | Nitroisobutylglycerol Trinitrate from nitromethane and formaldehyde...
CH3-NO2 + 3 CH2=O --> (HOCH2)3C-NO2
(HOCH2)3C-NO2 + 3HNO3 --> (O2NOCH2)3C-NO2 + 3H2O
A very powerfull OB balanced HE... |
Agreed, but the nitro group lowers its stability significantly and although its density exceeds that of nitroglycol, the much lower viscosity of the
latter gives it the VoD of a denser material!
And nitroglycol is a very good gelatiniser for NC, working at ordinary temps, unlike nib-glycerine trinitrate which is much slower even than NGL . . .
Nitroglycol's synthesis too, is easier if you have access to the glycol!
|
|
|
| Pages:
1
..
5
6
7
8
9
..
23 |