| Pages:
1
..
5
6
7
8
9
..
18 |
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
| Quote: | I never use carbonate.
I just wash the crude till no detectable acid in it, then dry, dissolve in methanol, add .3% urea, then crash in a water .01% urea mixture, filter and
dry |
OK so THAT'S what you mean. I can work with that. I didn't want to use carbonate because using that caused the ETN to form into a fluff thing that was
really annoying to get rid of. I'm glad to know I'm not the only one who wants to avoid carbonate with ETN cleaning.
And you used methanol. Would acetone work just as fine? I can use methanol if it is better, or just because you used it to greater effect.
An unrelated question: When recrystalizing urea from cold packs, is using methanol as good as ethanol during the process? Is methanol the same as
methylated spirits?
| Quote: | ManyInterests:
That's nice news. CHP works as a mono material. The outlet pressure is lower than ETN, but CHP is strong enough to initiate anything. This allows you
to get rid of compatibility problems with 2 or more substances in one cavity. I recommend CHP first. When wet, it has a beautiful blue, monochromatic
color. And he's non hygroscopic. Every success in the field of energetic materials causes a strong emotional experience. As a bonus, it's knowledge of
precise procedures that few people master.
Which is a big advantage in case of war... |
That's also good. A mono material is good because if I only needed to use one thing, then I would not have to worry about degradation. The ETN that I
made did not degrade at all when I left it in storage, only when it came in contact with the NHN. I will still make ETN because it's fun to make and a
decent energetic on its own. For CHP I need a whole different set of materials. But your videos are very clear and I only need a little more
equipment. The hardest thing will be making ammonium perchlorate. The only chlorate I made is potassium chlorate, but I will make sodium chlorate as
soon as the weather gets good in my area.
I also totally feel you with the emotional experience. One of the reasons why I wanted to get into energetics is to conquer some anxieties that I have
(I have an anxiety disorder) and I have to admit, just handling dangerous chemicals, producing them, and experimenting with this stuff really boosted
my confidence. I will need to gain more confidence in using the blasting caps in the 5 gallon buckets to make sure they go off correctly.
I still do want to make NHN/ETN caps, only because I discovered a problem that I absolutely want to fix. Or at least give it my best to fix. It is
fixable because I am not the only one to use NHN/ETN together, but I am the only one who mentioned a degradation of the ETN. It is possible that I am
the only one who noted that because I am the only one who didn't use the cap right away after making it, or the only one who used a transparent pen
body so you can see what is happening inside.
But just one question. NHN is known to have a detonation velocity of 7000 Meters per second (same as TNT, but it has a O-balance of -5). You did
mention the blast pressure in your LL8 thread, but would a 1 gram or 1.5 gram cap of pure NHN be sufficient to set off something like TNT or RDX?
Edit: You said CHP is not hygroscopic? I thought you said it was in one place (I forgot where). That was actually something I found concerning about
it. But if it is not hygroscopic then... well, that's cool!
Edit2: I saw your CHP video again. I noticed you used 25% ammonium hydroxide. I might be putting an order of 28%. when using it, should I use a little
less? Or the same amount? Would a large amount of 6% ammonium hydroxide (household ammonia) work as well?
| Quote: | | Yes, but in the case of war we are talking about IR drones that can track you and tanks with 1meter of armor. War is no longer a place for amateurs.
The Taliban only succeeded because we let them, petty politics you might. |
Don't get me started on the Taliban and the Afghan war. I can go on forever about how the whole situation sucks.
But I will mention one thing. Being an 'amateur' energetics maker is only useful in a war if you have an entire large group working with you. Making a
dozen grams or three of energetics for fun and giggles and intellectual curiosity is one thing. Making many, many kilograms or tons of the stuff is
something else entirely. You've seen how nitric acid is made on an industrial level... they have the distillation columns so big they are bigger than
the building I live in. (pictured below).
I mean to make a 1.3 kilogram block of ETN based plastic explosive you'll need 1 gallon of 68-70% of nitric acid and even more of sulfuric acid. I
made these calculations based on the proportions I used in making my ETN. Can you imagine how much time it'll take to make that? Or the difficulty in
one go? Imagine something going wrong, like a runaway... with that much acid.
When the Taliban used IEDs they used far more commercially made explosives, or at least massive amounts of 48-0-0 ammonium nitrate (which is a
controlled chemical where I live and cannot be obtained easily) as well as potassium chlorate (also a controlled chemical) when they tried to control
the ammonium nitrate. They didn't make it either, they bought it from Pakistan by the ton(s). This is also not considering the sheer amount of UXB
(unexploded ordinance) that was in the country from decades of war that was repurposed for IEDs.
War fantasies are fantasies. Nice to have a fantasy of being Rambo or John Wick or whatever every once in a while, but in reality I would rather just
be boring old me doing a boring day job and doing my other hobbies. I used the usename ManyInterests because... well, I have many interests!
Energetics is just one of them.

[Edited on 24-2-2022 by ManyInterests]
[Edited on 24-2-2022 by ManyInterests]
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
No, methylated spirits is generally ~95% ethanol and ~5% water it also contains a little denatonium benzoate, methyl isobutyl ketone and fluorescein
as denaturant. However, that is just in my neck of the woods, in other parts of the world it does contain methanol at varying concentrations, but to
my knowledge methanol is never the majority of the product. Check the SDS of the local brand available to you.
Quote: Originally posted by ManyInterests  |
I might be putting an order of 28%. when using it, should I use a little less? Or the same amount? Would a large amount of 6% ammonium hydroxide
(household ammonia) work as well?
|
Don't use household it is likely to contain other undesirable compounds. Why not make it yourself? React sodium hydroxide with ammonium sulfate or
urea and direct the generated ammonia gas into deionised water.
[Edited on 24-2-2022 by B(a)P]
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ManyInterests  |
OK so let me see if I understand you correctly. After the nitration is complete, I prepare the beaker/jar of distilled water with bicarbonate and urea
added in, then pour the nitrating mixture inside and filter as normal? And after drying, after dissolving all the ETN in warm acetone, I also add a
touch of urea to that as well?
|
[Troll]
Yeah, pour your nitration mixture in baking soda... Share pictures of the aftermath if you do
[/Troll]
Dissolve your crude product in acetone. Pour the hot solution in cold water with stirring.
It is THAT cold water where you add urea or NaHCO3 (with lots of stirring)
You can also add a pinch of baking soda directly in the flask with acetone if you've left a bit of water in your crude product.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
| Quote: | | No, methylated spirits is generally ~95% ethanol and ~5% water it also contains a little denatonium benzoate, methyl isobutyl ketone and fluorescein
as denaturant. However, that is just in my neck of the woods, in other parts of the world it does contain methanol at varying concentrations, but to
my knowledge methanol is never the majority of the product. Check the SDS of the local brand available to you. |
I see what you mean. OK that one is available in my area. It's mildly annoying to find since it isn't at most pharmacies, but I did manage to get
several bottles of 95% ethanol (denatured) that I did use in the past for urea recrystalization and hydrazine freebasing, as well as cleaning lab
equipment. I am well stocked for now.
| Quote: | | Don't use household it is likely to contain other undesirable compounds. Why not make it yourself? React sodium hydroxide with ammonium sulfate or
urea and direct the generated ammonia gas into deionised water. |
I originally wanted to do that, but looking at videos of people doing it had me think i needed more distillation gear that I didn't have. But then I
did see someone with a normal distillation setup and just hooked up a tube to the vent near the receiving flask and connected that one to a
water-filled flask in an ice bath to capture the ammonia gas. He ended up with a nice quantity of around 25% ammonia.
I think after seeing that I will have a go at it. It'll not only help in making CHP, but I think I'll try a hydrazine sulfate synthesis with a
different method than the Hoffman degradation of urea.
You mentioned using urea and sodium hydroxide. How much of each (proportions please!) Do I need to add water to the mix? Will urea that comes directly
from the cold pack suffice, or do I need to recrystalize it first?
| Quote: | [Troll]
Yeah, pour your nitration mixture in baking soda... Share pictures of the aftermath if you do
[/Troll]
Dissolve your crude product in acetone. Pour the hot solution in cold water with stirring.
It is THAT cold water where you add urea or NaHCO3 (with lots of stirring)
You can also add a pinch of baking soda directly in the flask with acetone if you've left a bit of water in your crude product.
|
I did that once... I should have shown the picture! It was frothier than a milkshake! That attempt was also me using KNO3 and not HNO3 to make the
nitrating mixture. I am not doing that again.
OK that is very clear. The next time I make a batch of ETN I'll do it that way. As well as wash the ETN with as much distilled water as possible
before hand. I usually dry my ETN as much as I can first, but I never touch it with my bare hands, so I don't know how dry it is to the touch, but it
did have the consistency of fine powder and didn't clump or stick.
Also... acetone or methanol? Which would you recommend in this case? Also when heating it, I assume it is to a mild 50C? It only took a few drops of
room temp acetone to dissolve my ETN, but that was because I made tiny little batches.
In other news... I did some thinking on how to solve the degradation problem of my NHN/ETN caps if it did turn out that they'll degrade no matter how
pure they are... I was thinking of maybe putting a small layer of plastic film between the ETN and the NHN in the cap? One that is so thin that it
won't impede the explosive train, but still prevent them from touching one another and possibly having that effect. Or another idea: Using a cap
within a cap. Since I am using pen bodies, I noticed that some pen caps can fit easily in other pen caps. So I could have the ETN in one cap and
insert a cap of NHN in it. The NHN would already detonate and set off the ETN, completing the explosive train.
Or even putting the NHN in a squib, like a Christmas light and putting a thin layer of tape on top to hold it in. I would need a little more practice
in making Christmas light igniters because they never seem to work for me.
What do you think?
[Edited on 25-2-2022 by ManyInterests]
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by ManyInterests  |
You mentioned using urea and sodium hydroxide. How much of each (proportions please!) Do I need to add water to the mix? Will urea that comes directly
from the cold pack suffice, or do I need to recrystalize it first?
[Edited on 25-2-2022 by ManyInterests] |
Configure your setup.
Add water, urea and sodium hydroxide in the following ratio 20:9:12 by weight into your reaction vessel. Almost no ammonia will be generated until
heat is applied. Keep the vessel receiving the ammonia in an ice bath.
To help you work out your yield ect the reaction is
CO(NH2)2 + 2NaOH --> 2NH3 + Na2CO3
Urea from a cold pack is fine, I wouldn't bother with recrystallisation.
|
|
|
katyushaslab
Hazard to Self
 
Posts: 81
Registered: 19-1-2021
Member Is Offline
Mood: precipitating
|
|
Quote: Originally posted by ManyInterests  |
Or even putting the NHN in a squib, like a Christmas light and putting a thin layer of tape on top to hold it in. I would need a little more practice
in making Christmas light igniters because they never seem to work for me.
What do you think?
[Edited on 25-2-2022 by ManyInterests] |
The filament in incandescent Christmas tree bulbs is absurdly fragile, and completely unsuited for safety critical applications such as initiation
systems.
Wire-wrapped/crimped nichrome is far superior in terms of reliability.
You can even solder nichrome to copper with a good acidic flux (to remove the oxide layer), though that is a complete pain in the ass. Phosphoric acid
based fluxes are what you want for this.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
I was building a dedicated spot welder for my bridge wires, it got back burnered with the coming of winter and a new power plant getting built, really
need to finish that thing!
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ManyInterests  |
OK that is very clear. The next time I make a batch of ETN I'll do it that way. As well as wash the ETN with as much distilled water as possible
before hand. I usually dry my ETN as much as I can first, but I never touch it with my bare hands, so I don't know how dry it is to the touch, but it
did have the consistency of fine powder and didn't clump or stick.
|
No need to use distilled water unless your tap water is weird. ETN is not going to react with anything in it.
Also, by wet I mean straight out of the filter paper. You need a bit of water to dissolve the NaHCO3. This implies you cant weight your product just
yet since it contains water.
Quote: Originally posted by ManyInterests  |
Also... acetone or methanol? Which would you recommend in this case? Also when heating it, I assume it is to a mild 50C? It only took a few drops of
room temp acetone to dissolve my ETN, but that was because I made tiny little batches.
|
When crashing in water use acetone. It is not "too good of a solvent" in this case since you can litterally use as much water as you want: ETN is
insoluble in water.
Heating is probably not necessary with acetone.
If you are playing with crystal size / shape by letting cool a solution of ETN in solvent, methanol will be more comfortable but there's no point of
doing that to ETN that hasnt been properly freed from acid by a couple of recrystallizations from acetone / water.
Lower 50's are your target because of ETN melting point. Avoid some butt clenching moment by thinking ahead carefully.
Quote: Originally posted by ManyInterests  |
In other news... I did some thinking on how to solve the degradation problem of my NHN/ETN caps if it did turn out that they'll degrade no matter how
pure they are... I was thinking of maybe putting a small layer of plastic film between the ETN and the NHN in the cap? One that is so thin that it
won't impede the explosive train, but still prevent them from touching one another and possibly having that effect. Or another idea: Using a cap
within a cap. Since I am using pen bodies, I noticed that some pen caps can fit easily in other pen caps. So I could have the ETN in one cap and
insert a cap of NHN in it. The NHN would already detonate and set off the ETN, completing the explosive train.
|
If you look at cutaways from military fuzes and ordnance you'll see a lot of cardboard, bakelite, copper or brass washers used even when you'd think
there is no chemical incompatibility so that's probably a good idea anyway.
You could preform Al foil and use it as a capsule to lower your NHN in your pen body or something along those lines.
In your case I'm really curious about what's happening. Is it real degradation of the product or could it just be the mixing of two relatively loose
powders that give your that impression ?
In short: is it a chemical reaction or just a visual effect ?
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
electric ignitor
For the electric ignition, an ordinary wire from internet and a wire scourer for the dishes are enough. It is made of flat wire. The core is steel
and the surface is nickel. Resistance is cca 0.2 Ohm. And it works absolutely reliably. The voltage is usually 12 V / 10A. It can only be 6 V / 5 A.
Or 10 V / 20 A. Reliable function is not affected and works in large range of voltage and current. Everything else is in the picture.

Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
| Quote: |
No need to use distilled water unless your tap water is weird. ETN is not going to react with anything in it.
Also, by wet I mean straight out of the filter paper. You need a bit of water to dissolve the NaHCO3. This implies you cant weight your product just
yet since it contains water. |
I use distilled water as a matter of principle when doing any chemistry stuff. It is really cheap. I don't think there's anything up with my tap
water, but you know what I mean.
Also how I do it is when I wash out the ETN and dry it, I keep it on a waterproof surface (plastic wrap, styrofoam plate, etc) put paper towels below
the coffee filter holding the ETN and more paper towels over it to both absorb the water on top and to prevent any dust from coming onto the ETN. I
keep it like this for a day or so until the water evaporates. After that I weigh the produce and commence with recrystalization.
| Quote: |
When crashing in water use acetone. It is not "too good of a solvent" in this case since you can litterally use as much water as you want: ETN is
insoluble in water.
Heating is probably not necessary with acetone.
If you are playing with crystal size / shape by letting cool a solution of ETN in solvent, methanol will be more comfortable but there's no point of
doing that to ETN that hasnt been properly freed from acid by a couple of recrystallizations from acetone / water.
Lower 50's are your target because of ETN melting point. Avoid some butt clenching moment by thinking ahead carefully. |
Gotcha. I'll continue to use room temp acetone then. Now that I think about it I think there might have been acid left over in my ETN because I kept
filtering the ETN until the coffee filter became saturated and then took it away. I think next time I will only filter little bits of the ETN at a
time and just wash it with water until I am confident that that bit is acid-free before replacing the coffee filter.
| Quote: | If you look at cutaways from military fuzes and ordnance you'll see a lot of cardboard, bakelite, copper or brass washers used even when you'd think
there is no chemical incompatibility so that's probably a good idea anyway.
You could preform Al foil and use it as a capsule to lower your NHN in your pen body or something along those lines.
In your case I'm really curious about what's happening. Is it real degradation of the product or could it just be the mixing of two relatively loose
powders that give your that impression ?
In short: is it a chemical reaction or just a visual effect ?
|
It is definitely a chemical reaction of some sort and not a visual effect. It started out a little bit but eventually all the ETN turned into the
sawdust-brown color you see. When I detonated my first cap that had all-white ETN the power was significant. When I set off a second one (with equal
amounts ETN and NHN) it was barely noticeable. Also when I detonated a large cap with 1 gram of ETN and 0.4 grams of NHN, the ETN was fully brown and
the power was near non-existent. Something is up.
I don't think it is the ETN alone. Because when I disposed of my current supply of ETN, it was still very snow-white.
I need to mention that when I made my NHN, the nickel nitrate that I used was slightly acidic. I discussed it with someone in another thread and
showed the litmus paper reading. After diluting it slightly I did see an improvement, but still slightly acidic. The guy said it was OK. My NHN
synthesis was a success, and while I didn't have the chance to wash the NHN with more water and ethanol (95%) I did get around to doing that later.
But giving the NHN a water and ethanol wash didn't really change much.
For my next attempt in the coming fall I will be washing out the NHN with water and alcohol the first time around. Several washes and filter only bits
a time so the paper doesn't get clogged like my first attempt.
After I clean the hell out of them, I want to make one cap with both powders touching and another with some kind of separator (I will need a bit of
practice with things like sugar and flour to make sure I get it right before loading up a cap with energetics) and monitor them for a month. If
neither degrades then I think I got me a winner synthesis. If he unseparated one degrades regardless, then I'll just have to make caps with
separators.
I did see videos from WW2 showing the production of military explosives that did show detonators and boosters being made separately and put in
separate containers. I think I also did see that in some cutout that had the booster in a separate cup-like container
| Quote: | | For the electric ignition, an ordinary wire from internet and a wire scourer for the dishes are enough. It is made of flat wire. The core is steel
and the surface is nickel. Resistance is cca 0.2 Ohm. And it works absolutely reliably. The voltage is usually 12 V / 10A. It can only be 6 V / 5 A.
Or 10 V / 20 A. Reliable function is not affected and works in large range of voltage and current. Everything else is in the picture.
|
| Quote: | The filament in incandescent Christmas tree bulbs is absurdly fragile, and completely unsuited for safety critical applications such as initiation
systems.
Wire-wrapped/crimped nichrome is far superior in terms of reliability.
You can even solder nichrome to copper with a good acidic flux (to remove the oxide layer), though that is a complete pain in the ass. Phosphoric acid
based fluxes are what you want for this. |
I've become pretty adept at making electrical matches from my own design. I use wires that I got from an electronics supply store. I experimented
extensively with nichrome wire of various thickness. I used 0.04mm 0.08mm 0.1mm and 0.25mm (I ordered 0.20mm but it hasn't arrived yet)
My verdict is that the 0.25mm is the best of the bunch. I managed to light up a match (an actual match) using 2 AA batteries. So a simple 9v battery
would be sufficient for most applications, while a 9v battery using 6 AA batteries (they sell those packs) is all but guaranteed to light it up, I use
12v AA battery packs or even a C-cell battery pack which is overpowered, but that is a good thing (I have 12V D-cell batteries, but that is going too
far I think).
The 0.04mm is beyond worthless. When lighting up the nichrome wire that is attached to the battery will burn out almost immediately and not carry the
current to the match to the ignition mixture. The 0.08mm can work, but it needs more care. I need to cut out a large length of wire, much more than
the 0.25mm and it is very bouncy and moves around. I noticed that to make a more reliable match I need to use paper matches (as opposed to a single
wooden match with 0.25mm) and put the wire between them first before wrapping it around. Even then it can still come loose and have reliability
issues.
The 0.10mm is better than the 0.08 because it is slightly thicker, but still runs into the same problem.
None of the matches that I used and inserted into my caps failed to go off, so I know they work. I also coated them with homemade nitrocellulose
lacquer (I dissolved some NC in acetone and coated the match head with it to fix the nichrome in place). The last cap I set off was interesting in
that the actual match did not burn, but the NHN immediately went off when the wire started to heat up.
I agree that Christmas lights are basically worthless. The only advantage of a Christmas light is that it needs next to no voltage to work. I did make
a test squib with ground match heads in it and I lit it up with a CR2032 battery.
Also I got something to show off. I found metal tissue box at a discount store. Would this make for a good witness plate? It is really thick and very
solid metal.
 
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Is it steel ? Looks a like plast. Thickness I estimate on 6 - 6.5 mm according hands. Is it too much for any charge 300 - 1000mg. I recommend
pour into cavity the lead of high 30 - 40 mm. After you will have good target for relativey exact measurement of charges 300 - 1000mg of any EM. The
surface can be used for 5 test craters. And after heating again and again repeatedly. Good test box. For direct penetration is this box too thick. Is
nonsense destroy it. I'm still waiting for your holes or craters ...
I haven't seen one yet ....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
Yes it is steel! But you won't be seeing any holes or craters for a while. Like I said, I've put aside the chemistry kit and it will be months before
I return to it. While I do post on a frequent basis (for now) I'm just gathering info for the moment.
I will drop you a PM (in several months or even in a year at most) once I finally return to this and start blowing holes into the steel box I got). I
think I will have a much better setup in the future to blow holes while also muffling the sound.
| Quote: | Configure your setup.
Add water, urea and sodium hydroxide in the following ratio 20:9:12 by weight into your reaction vessel. Almost no ammonia will be generated until
heat is applied. Keep the vessel receiving the ammonia in an ice bath.
To help you work out your yield ect the reaction is
CO(NH2)2 + 2NaOH --> 2NH3 + Na2CO3
Urea from a cold pack is fine, I wouldn't bother with recrystallisation. |
That sounds good, so 20 grams water, 9 grams urea, 12 grams sodium hydroxide adjusting upwards of course.
This is how my setup would look like. https://youtu.be/LiJ3Z8a6Ld0
Basically a simple distillation setup, but the gas is routed to another water filled flask in an icebath. the only condenser I have is a long Alihn
consender, but it shouldn't affect it much (or maybe even have a better effect.
|
|
|
Rakunin
Harmless

Posts: 5
Registered: 11-3-2022
Member Is Offline
|
|
How much Mercury Fulminate will be equivalent to a No. 8?
|
|
|
katyushaslab
Hazard to Self
 
Posts: 81
Registered: 19-1-2021
Member Is Offline
Mood: precipitating
|
|
> No. 8 test caps contain the equivalent of two grams of a mixture of
80 percent mercury fulminate and 20 percent potassium chlorate
source: https://www.nps.gov/parkhistory/online_books/npsg/explosives...
|
|
|
Rakunin
Harmless

Posts: 5
Registered: 11-3-2022
Member Is Offline
|
|
Thanks for the quick response and elaborate source.
Will it be sufficient to detonate AN, ANFO or UN, UNFO - or is a booster necessary?
|
|
|
katyushaslab
Hazard to Self
 
Posts: 81
Registered: 19-1-2021
Member Is Offline
Mood: precipitating
|
|
Probably won't set off ANFO, you would want a booster. Might set off urea nitrate though, or an Ammonal mix.
Why would you mix urea nitrate with fuel oil though? I don't think I have seen that done before.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by Rakunin  |
Thanks for the quick response and elaborate source.
Will it be sufficient to detonate AN, ANFO or UN, UNFO - or is a booster necessary? |
Are you sure you mean UNFO? UN is already OB negative. Urea is sometimes introduced into ANFO to help with its stability in damp or hot environments
in the hard rock metal sulfide ore mining sector, maybe this is where the confusion lies?
|
|
|
Rakunin
Harmless

Posts: 5
Registered: 11-3-2022
Member Is Offline
|
|
Quote: Originally posted by katyushaslab  | Probably won't set off ANFO, you would want a booster. Might set off urea nitrate though, or an Ammonal mix.
Why would you mix urea nitrate with fuel oil though? I don't think I have seen that done before. |
Quote: Originally posted by B(a)P  | | Are you sure you mean UNFO? UN is already OB negative. Urea is sometimes introduced into ANFO to help with its stability in damp or hot environments
in the hard rock metal sulfide ore mining sector, maybe this is where the confusion lies? |
No, you're right. I do not know why I wrote it, it was a mistake. Actually, I was more interested in the first one. I have never had problems with
commercial det caps and AN mixture or ANFO, but have often used a piece of detcord of approx. 10 cm to be sure, but without has worked fine. But
thanks for the reply and sorry for the confusion.
But now I will try to try with the above cap. I will come with an update and result.
[Edited on 17-3-2022 by Rakunin]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
WAHEXAN
A very sensitive AN-based compound was once developed at the Liptakov Laboratory. Partly resistant to moisture, sensitive to only 300 mg HMTD
With a critical diameter of only 20 mm. The preparation requires a fairly long process. The ideal density is around 1.0 g / cc.
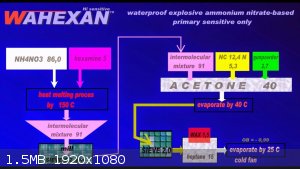
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by Rakunin  |
Actually, I was more interested in the first one. I have never had problems with commercial det caps and AN mixture or ANFO, but have often used a
piece of detcord of approx. 10 cm to be sure, but without has worked fine. But thanks for the reply and sorry for the confusion.
But now I will try to try with the above cap. I will come with an update and result.
[Edited on 17-3-2022 by Rakunin] |
Please do report back, I would be curious to know. I have had limited exposure to ANFO, but when I have seen it used it is with a TNT primer or det
cord.
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
| Quote: | Thanks for the quick response and elaborate source.
Will it be sufficient to detonate AN, ANFO or UN, UNFO - or is a booster necessary? |
Is UN urea nitrate? What is UNFO.
I am not experienced with ANFO, but I do believe that ANFO is fairly insensitive and that a booster is necessary. The commercial caps you used, are
they no. 8s or compound detonators?
While I have no interest in ANFO, I am still curious about your results.
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ManyInterests  | | Quote: | Thanks for the quick response and elaborate source.
Will it be sufficient to detonate AN, ANFO or UN, UNFO - or is a booster necessary? |
Is UN urea nitrate? What is UNFO.
I am not experienced with ANFO, but I do believe that ANFO is fairly insensitive and that a booster is necessary. The commercial caps you used, are
they no. 8s or compound detonators?
UNFO is made up
While I have no interest in ANFO, I am still curious about your results. |
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Usually the same companies that deliver / manufacture AN based explosives for mining also sell boosters that are quite big.
16 grams of plasticized PETN will certainly help set off anything I can think of.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
Quote: Originally posted by Herr Haber  | Usually the same companies that deliver / manufacture AN based explosives for mining also sell boosters that are quite big.
16 grams of plasticized PETN will certainly help set off anything I can think of.
|
Yeah, that sounds about right. I did hear about some compound detonators that did have 14 to 16 grams of PETN as a booster.
But I did think about something earlier. As a plasticizer for energetics. Would the rubber used to manufacture chewing gum also work as a plasticizer?
There was a thread on this forum from 2006 where someone did talk about it, and I did do some research into gum bases. While most chewing gum
manufacturers use a different polymer for their product (if you chew gum from several makers and notice subtle differences, that's why) and they tend
to guard their exact composition jealously.
Would they work?
Also when shopping, both in hardware stores and online, most self amalgamating tape I see (except for the Scapa 2501) is silicone. I am guessing that
silicone is not PiB? Would it work to plasticize energetics? I also read about lithium grease being used to that effect as well, but I am not sure.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
There are several threads on the forum and videos on Youtube about extracting PIB from gloves or self amalgamating tape.
But yeah, I too thought about chewing gum. It's probably a great idea. Washing the sugars / polyols / talcum from the surface is easy.
I've only heard of PIB used in chewing gums (but havent looked into it seriously). I guess freezing the tablets prior to crushing them into small
pieces would help the solvent a lot.
It would be worth documenting if someone tried extracting PIB from chewing gum.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
| Pages:
1
..
5
6
7
8
9
..
18 |