| Pages:
1
..
62
63
64
65
66
..
77 |
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Lovely, Pok. Thanks for these.
(subscribed.)
[Edited on 4-3-2019 by j_sum1]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by mayko  | Hot off the press, a never-before-published photograph of plutonium tetrafluoride! This was obtained by nuclear anthropologist Martin Pfeiffer via
FOIA. It's a little disappointing (it is known as 'pink cake'; this is apparently misleading.) but upthread there are some very nice pictures of
exotic things like plutonium trichloride
If you're on twitter and interested in nuclear history/technology/sociology, definitely check him out!
|
Martin has a new batch of pictures, probably the only of this plutonium compound in the public record.

Announcement:
https://twitter.com/NuclearAnthro/status/1107414103499276288
More here, under My FOIA Responsive Documents/PuF4 Plutonium Tetrafluoride Pictures/
https://osf.io/46sfd/
Some chemical background:
https://www.dropbox.com/s/829rbeoruo3a78w/Preparation%20plut...
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
100%?! did they really manage to remove every atom of impurites?!
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
To three significant figures, why not?
Now if he had said, 100.00000000000%, I would be suspicious.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
No, they just rounded off to the nearest whole percentage.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
yesterday (a few hours ago) was my birthday, my best friend gave me this as a gift, Autunite sample, i've been looking for one one ebay for years,
this made me so happy
(crappy photos taken with phone, i'll take better photos with a real camera in the morning, just came home after the party hahahhaa)
  
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Wow, that's a beautiful sample, I'd love to len one, too  . .
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
It just means it is between 100.5% and 99.5%, obviously it can't be more than 100, so it is 99.5 or higher. Just like the other one which is between
93.5 and 94.5%.
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
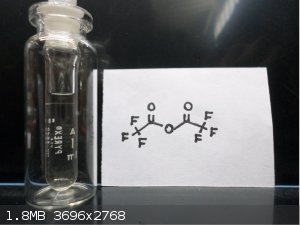
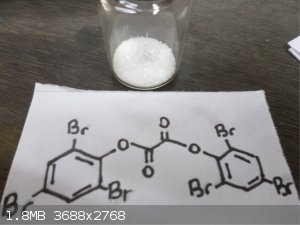
Here's some Trifluoroacetic anhydride  and bis(2,4,6 Tribromophenol) Oxalate and bis(2,4,6 Tribromophenol) Oxalate

|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
KMnO4 solution 
[Edited on 26-3-2019 by greenlight]

[Edited on 26-3-2019 by greenlight]
Be good, otherwise be good at it 
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
HgCl2 after a broken mercury thermometer dissolved in an excess of HCl left for a month.

|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Yikes greenlight, you really should neutralize stuff like that instead of pouring straight down the drain! I know it probably wasn't
much, since KMnO4's color is so intense, but in this case it's easy: just add sugar!
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by MrHomeScientist  | | Yikes greenlight, you really should neutralize stuff like that instead of pouring straight down the drain! I know it probably wasn't
much, since KMnO4's color is so intense, but in this case it's easy: just add sugar! |
It would decompose in minutes when mixing with dirty drain waters.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
some iodine in an evauated and sealed test tube (while cold it is transparent with solid iodine on the walls)

---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by MrHomeScientist  | | Yikes greenlight, you really should neutralize stuff like that instead of pouring straight down the drain! I know it probably wasn't
much, since KMnO4's color is so intense, but in this case it's easy: just add sugar! |
Yeah I dont usually just pour stuff down the drain but it was a 0.02M solution and the colour is indeed intense even with very small amounts of
solute.
Be good, otherwise be good at it 
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
I found a picture from years ago from my first reaction ever, I must have had no clue what I was doing. I had seen a video showing copper sulfates
reaction with bases and figured since baking soda was a base, it would react with the copper sulfate. I must have been about 12. Now that I think of
it, the beautiful colors of copper compounds are really what got me interested in chemistry all of those years ago.

[Edited on 4-7-19 by Abromination]
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Wizzard1
Harmless

Posts: 10
Registered: 13-2-2019
Member Is Offline
|
|
By-product of Neodymium Sulfate purification. Enjoy!! It was about 3" across.

|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
So is it FeSO4 or Pr2(SO4)3?
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Sometimes the simple things are most satisfying.

It is nice to be back in the lab.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Beautiful, Wizzard1! I'm quite familiar with those from all my magnet work a while back. Mine never stayed nice for long.
It's FeSO4, fusso. You get tons of it from magnet processing. Pr is in such small quantities you'd need a hell of a lot of magnets to make
that much, not to mention separating and purifying it from the Nd.
|
|
|
bipolar
Harmless

Posts: 24
Registered: 24-3-2019
Member Is Offline
|
|
Polycrystal of 5-MeO-DMT freebase :3

This beauty weighs about 380 mg 
Made it from melatonin (1. hydrolysis; 2. reductive amination with formaldehyde / NaBH4).
[Edited on 21-4-2019 by bipolar]
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by bipolar  | Polycrystal of 5-MeO-DMT freebase :3
This beauty weighs about 380 mg 
Made it from melatonin (1. hydrolysis; 2. reductive amination with formaldehyde / NaBH4).
[Edited on 21-4-2019 by bipolar] |
Amazing crystal and job. Can you please provide full procedure? I would love to get back to organic chemistry as I have access to NaBH4 now. 
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Some crystals of salicylic acid, grown from toluene/hexane. i was *trying* to make butyl salicylate, but I got these instead.

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Are you sure that's salicylic acid? When I recrystallised some of mine, I got needle-like crystals. Admittedly, I don't recall which solvent I used.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by DavidJR  | | Are you sure that's salicylic acid? When I recrystallised some of mine, I got needle-like crystals. Admittedly, I don't recall which solvent I used.
|
The melting point checked out (although I didn't do a mixed one). From water, I usually see needles.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
..
62
63
64
65
66
..
77 |