| Pages:
1
..
62
63
64
65
66
..
104 |
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
You may think water/ice is boring, but it works well. Measure the mass of some warm water, add some ice at 0 oC in an insulated container, measure
the final temperature and total mass.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Yeah except that is not the experiment I had in mind. I am less interested in a quantitative calorimetric measurement than a good visual
demonstration of phase change. A flat line on an otherwise decreasing curve should do this well. We will do some calorimetry later (perhaps) and
melting ice as you describe is a likely candidate.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Fair enough.
Glacial acetic acid has a convenient melting point for such work, but it tends to supercool, and the liquid's a bit nasty. Cyclohexanol melts around
room temperature, and could work.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
In theory it does, but it almost always contains
a trace amount of water which depresses its melting point significantly.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by zts16  | | In theory it does, but it almost always contains
a trace amount of water which depresses its melting point significantly. |
I haven't had that problem- in our lab, we add about 2% cyclohexanone to the damned stuff so that it doesn't keep freezing on us.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I did it with tert butanol. We got a nice sudden flattening of the cooling curve but did not get a noticeable restart of the curve after it had all
solidified. Next time I will use a colder ice bath. But also the thermal conductivity and heat transfer is going to be different for a solid.
I would love to do this with sodium but cannot think of a good way of doing it. Gallium will shatter glass. Maybe lead or a solder? I am
intuitively guessing that metals will show the property a bit better. Anyway, it did work but there is room for improvement.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by j_sum1  | | Maybe lead or a solder? I am intuitively guessing that metals will show the property a bit better. Anyway, it did work but there is room for
improvement. |
The trouble with a solder or other mixture is that you'd have to use the eutectic to get a sharp melting point rather than a range.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
ElizabethGreene
Hazard to Others
  
Posts: 141
Registered: 15-10-2012
Member Is Offline
Mood: No Mood
|
|
Palladium vs. NaOH
Will a hot (75C) solution of NaOH and water attack Palladium?
Related question, how did you find this answer?
Thanks.
|
|
|
Texium
|
Threads Merged
20-10-2016 at 12:44 |
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I would think not. The palladium would have to form some kind of oxoanion and there are no signs of palladium oxoanions existing.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I have two oxygen concentrators, one I keep as backup for the one with my mother,
I occasionally use it for myself, and occasional for experimenting 
one of these http://www.ebay.co.uk/itm/GENUINE-JYT-90-ADJUSTABLE-1-5L-OXY...
specified at 1 l/min. @ 90% O2, 5 l/min. @ 40%.
they both work ... very good smouldering wood reaction.
Eventually the xeolite will loose efficiency and O2 yield will drop.
Can someone suggest an EASY oxygen concentration test that I can perform with minimal diagnostic stuff to carry ?
Just to ensure that oxygen is being concentrated.
The O2 is not for life support, just assistance, so non-critical, absolute measurement not required, just relative to new/now.
Separate question;
Out of curiosity I would like to know the absolute O2 concentration of the machine that I have,
I have burettes, flasks etc. but nothing specifically for handling gases. I can burn Na, Mg etc.
but prefer not to if a less energetic method such as bubbling through a solution guaranteed to absorb or react with ALL of the O2 can be
done simply.
I can think of many possible methods but has anyone any practical advice ?
P.S. my mother has been using it for many hours per day almost daily for over nine months, so I can recommend this model ... so far.
(although less than £200 when I bought them)
It can extend your active smoking life well into the eighties 
[Edited on 21-10-2016 by Sulaiman]
[Edited on 21-10-2016 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
See this paper, which uses oxidation of steel wool: http://pubs.acs.org/doi/abs/10.1021/ed1008798
It won't be very precise, but it seems easy.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I have watched YouTube videos and looked at several web pages,
I only have one 2 ml and one 30 ml syringe in ground glass,
so iron, copper etc. in a heated tube between syringes would require new gas syringes.
I'm not too keen on rusting/corroding/burning stuff in a burette but it seems my most likely route,
I would of course compensate for the volume of 'fuel'+support
and measure at ambient pressure in the burette ... not partial vacuum like most demonstrations 
I'd like something simple and reliable.
(I have methylene blue to detect oxygen, Thunberg tubes etc. if that helps with suggestions)
[Edited on 21-10-2016 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
I have some guanidine carbonate, but I would like to convert it to (dry) guanidine hydrochloride, for reactions involving freebase guanidine in
alcohols. Although guanidinium cations of strong acids should be stable in boiling water, I am worried that the extreme heat needed to dry the
hygroscopic guanidium salt would degrade it to ammonium chloride and urea. Is this concern misguided?
|
|
|
bolbol
Hazard to Others
  
Posts: 167
Registered: 3-1-2015
Member Is Offline
Mood: No Mood
|
|
Unknown purple solution
Today I was able to get a purple colored solution after mixing two different solutions.
I had this chunk of white solid(Either sodium salicylate or salicylic acid) from the a long time ago when I extracted aspirin from pills by acidifying
the solution and basifying it back and forth. I am not sure what I ended up with but it was not soluble in water. I tried dissolving it but wouldnt
work so I added some isopropyl alcohol to it to see if it helped. I saw a decrease in the amount of crystals on the bottom of the beaker but it didnt
fully dissolve..
At the same time I had a beaker on the hotplate that pretty much limestone dissolving in HCl. Most of the HCl was consumed so I was heating it up to
dissolve the last bit of the solid limestone chunk in it. This solution contained mostly calcium with small amounts of magnesium and iron(III) with
other trace elements. I decided to pour some of this into my beaker with the alcohol and water mixture and it turned dark purple as if it was suddenly
a permanganate solution.
Any ideas as to what the hell this could be? I don't know of any substance that has the same color as permanganates. Any insight would be appreciated.
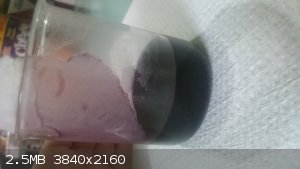
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Fe+3 ions from your limestone solution form purple (or other colors depending on the nature of the phenol) complexes with phenols (which salicylates
are).
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Yep, that is most definitely the iron(III) salicylate complex. It works very well as a qualitative test since the color is so rich.
|
|
|
Texium
|
Threads Merged
12-11-2016 at 11:51 |
bolbol
Hazard to Others
  
Posts: 167
Registered: 3-1-2015
Member Is Offline
Mood: No Mood
|
|
Oh thats interesting especially for me as I have no background in organic chem. Thanks!
|
|
|
ficolas
Hazard to Others
  
Posts: 146
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
Iron screw electrolysis
I wanted to make some iron oxide, so I electrolysed some rusted screw, however the solution turned a color I did not expect it to, so Im worried the
screws could contain chromium.
http://i.imgur.com/CNaUKG5.jpg
The screws were alredy rusted when I used them, so I didnt think they could be stainless steel.
The colors make some sense to me, however is now what I remeber from doing it another time. I just want to make sure because I really dont want this
to have carcinogenic compounds.
[Edited on 14-11-2016 by ficolas]
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Why so many acids yet few bases
When searching for chemicals I find a plethora of acids but very few bases - why ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Leaving aside the notion that every acid has its conjugate base...
I think it comes down to nomenclature as much as anything else. By which I mean that anything with a -COOH group gets (logically) designated as acid,
compounds with a -NH2 group are called amines even though they act as bases (generally).
I'll let some else speak for the inorganic side of things.
|
|
|
awlb2
Harmless

Posts: 27
Registered: 21-11-2016
Member Is Offline
Mood: No Mood
|
|
Hello, I would like to know if phosphoric acid or sodium bisulfate is a suitable catalyst for esterification of short chain alcohols(e.g. ethanol) and
aromatic carboxylic acids (e.g. benzoic acid)- I know esterifications occur 'slowly' using this catalyst but how much slower compared to a strong acid
such as H2SO4 or p-TsOH, is it still feasible ? Does anyone know what pKa is approximately needed to deprotonate the carboxylic
acid- does it simply have to be lower than that of the carboxylic acid's pKa. As sulphuric acid is not available for me(chemophobic parents) I am
looking for other strong, less corrosive, acid catalysts.
Thank you in advance if you are able to help!
[Edited on 21-11-2016 by awlb2]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Phosphoric acid should be a suitable substitute for sulfuric.
|
|
|
awlb2
Harmless

Posts: 27
Registered: 21-11-2016
Member Is Offline
Mood: No Mood
|
|
Thank you very much for replying Metacelsus- I will try it with phosphoric acid.
|
|
|
awlb2
Harmless

Posts: 27
Registered: 21-11-2016
Member Is Offline
Mood: No Mood
|
|
But what about sodium bisulfate? I believe it reacts with ethanol to form sodium ethyl sulfate- could this then react with the carboxylic acid to form
the ester or would it react with more ethanol to form diethyl ether?
|
|
|
Meltonium
Hazard to Self
 
Posts: 97
Registered: 23-9-2016
Location: Home in pajamas
Member Is Offline
Mood: Fluorinated
|
|
Gringard Question
I've always seen gringard reactions done with using bromine as the halogen with the magnesium. Can the reagent be prepared using chlorine or iodine?
Are there significant drawbacks to using something other than bromine?
|
|
|
Texium
|
Threads Merged
22-11-2016 at 20:16 |
| Pages:
1
..
62
63
64
65
66
..
104 |